Beruflich Dokumente
Kultur Dokumente
Virtual Lab - Gas Laws Simulation Instructions
Hochgeladen von
AtharrizwanCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Virtual Lab - Gas Laws Simulation Instructions
Hochgeladen von
AtharrizwanCopyright:
Verfügbare Formate
Virtual Lab: Gas Laws
Observations from performing virtual experiment website:
Website: https://teachchemistry.org/classroom-resources/the-gas-laws-simulation
Make sure you are on the corresponding tab for the gas law in the simulation. The values for P1, V1,
and T1 are automatically entered. Use the arrows to change the indicated values.
Create a Word document and Give the file the following name: “Gas Law Virtual – Your Name”. You will have
to upload this file to eLearn once you have complete the virtual lab. Make sure to copy and answer the question
after completing each part into your Word document.
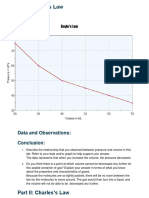
Part I: Boyle’s Law:
1. Notice that moles (dots in container) and temperature are held constant.
2. Change the volume, pressure, or both.
3. Make sure it creates a different point are not close together on the graph.
4. Click “Add Data”
5. Repeat steps 2-4 until the 5th point which you may have to click “Calculate” in the bottom box.
6. Right click the on simulation and copy image and paste into Word document.
Q1: What do you notice about the trend between changes in pressure and volume?
Part II: Charles’ Law:
1. Notice that moles (dots in container) and pressure are held constant.
2. Change the volume, temperature, or both.
3. Make sure it creates a different point are not close together on the graph.
4. Click “Add Data”
5. Repeat steps 2-4 until the 5th point which you may have to click “Calculate” in the bottom box.
6. Right click the on simulation and copy image and paste into Word document.
Q2: What do you notice about the trend between changes in temperature and volume?
Part III: Gay-Lussac’s Law:
1. Notice that moles (dots in container) and pressure are held constant.
2. Change the volume, temperature, or both.
3. Make sure it creates a different point are not close together on the graph.
4. Click “Add Data”
5. Repeat steps 2-4 until the 5th point which you may have to click “Calculate” in the bottom box.
6. Right click the on simulation and copy image and paste into Word document.
Q3: What do you notice about the trend between changes in temperature and pressure?
Das könnte Ihnen auch gefallen
- Graded Exam 1Dokument12 SeitenGraded Exam 1AtharrizwanNoch keine Bewertungen
- Equation of State of Ideal Gases With Cobra 4Dokument10 SeitenEquation of State of Ideal Gases With Cobra 4DanielLugoNoch keine Bewertungen
- ECNS 203 Quiz ThreeDokument8 SeitenECNS 203 Quiz ThreeAtharrizwanNoch keine Bewertungen
- Gas Laws WeberciseDokument7 SeitenGas Laws Weberciseapi-3652150540% (1)
- Simulation Gaslaws StudentDokument8 SeitenSimulation Gaslaws StudentSerenity WadeNoch keine Bewertungen
- Charles LawDokument17 SeitenCharles Lawkarijsd08Noch keine Bewertungen
- Lillian Frutos - Lab - Boyle's Law Using LabQuest (2021)Dokument2 SeitenLillian Frutos - Lab - Boyle's Law Using LabQuest (2021)Lillian FrutosNoch keine Bewertungen
- Gas Law ActivityDokument7 SeitenGas Law Activitydaniel tabuzoNoch keine Bewertungen
- Student Exploration: Boyle's Law and Charles' LawDokument7 SeitenStudent Exploration: Boyle's Law and Charles' Lawapi-280439402Noch keine Bewertungen
- Part 1: Boyle's Law: Pressure-vs-Volume: MaterialsDokument5 SeitenPart 1: Boyle's Law: Pressure-vs-Volume: MaterialsMadison IngramNoch keine Bewertungen
- Lab 1 - Ideal Gas LawDokument8 SeitenLab 1 - Ideal Gas LawJammy TanNoch keine Bewertungen
- Activity 4 Science 10 4th QDokument2 SeitenActivity 4 Science 10 4th QStarskie MonacarNoch keine Bewertungen
- Lab #52: Pressure and Volume Relationship in Gases-Boyle's LawDokument3 SeitenLab #52: Pressure and Volume Relationship in Gases-Boyle's LawSidemen For LifeNoch keine Bewertungen
- Boy Les Charles SeDokument14 SeitenBoy Les Charles SeLenny Morell ZayasNoch keine Bewertungen
- Heat Exchanger: Prerequisites: Before Beginning, The Users Need To Know How ToDokument4 SeitenHeat Exchanger: Prerequisites: Before Beginning, The Users Need To Know How Toعبد اللهNoch keine Bewertungen
- 6A. Gas LawsDokument3 Seiten6A. Gas Lawsreyeslucasmat4Noch keine Bewertungen
- Boyles & Charles' Law Simulation GizmoDokument7 SeitenBoyles & Charles' Law Simulation GizmoamyNoch keine Bewertungen
- Student Exploration: Boyle's Law and Charles's LawDokument14 SeitenStudent Exploration: Boyle's Law and Charles's LawStephanie ValienteNoch keine Bewertungen
- PH ETGas PropertiesDokument4 SeitenPH ETGas PropertiesRaven Bacudo NiemoNoch keine Bewertungen
- CWV 01 COMP Endothermic ReactionsDokument5 SeitenCWV 01 COMP Endothermic ReactionsCarlos Alberto MoviNoch keine Bewertungen
- GaslawsexplorationlabDokument5 SeitenGaslawsexplorationlabapi-320087626Noch keine Bewertungen
- Key - 8.1 Gas Law Lab PDFDokument6 SeitenKey - 8.1 Gas Law Lab PDFzhuzaiNoch keine Bewertungen
- Ideal Gas Laws - Explore Learning SimulationDokument19 SeitenIdeal Gas Laws - Explore Learning SimulationSandy RiveraNoch keine Bewertungen
- Importing Data Into MATLAB: Carbon Monoxide Vapor Pressure DataDokument2 SeitenImporting Data Into MATLAB: Carbon Monoxide Vapor Pressure DatamagiNoch keine Bewertungen
- Data and Observations: ConclusionDokument2 SeitenData and Observations: ConclusionStephany LeviNoch keine Bewertungen
- Bolyes Law PVRelationship Student ActivityDokument7 SeitenBolyes Law PVRelationship Student ActivityRicardo Esquivel SilvaNoch keine Bewertungen
- Bolyes Law PVRelationship Student ActivityDokument7 SeitenBolyes Law PVRelationship Student ActivityRicardo Esquivel SilvaNoch keine Bewertungen
- Volume Temperature RelationshipsDokument3 SeitenVolume Temperature RelationshipsAndrew SilvaNoch keine Bewertungen
- 1Dokument18 Seiten1Konul AlizadehNoch keine Bewertungen
- Bolyes Law PVRelationship Student ActivityDokument4 SeitenBolyes Law PVRelationship Student ActivityRicardo Esquivel SilvaNoch keine Bewertungen
- Assignment Week3Dokument20 SeitenAssignment Week3totomkosNoch keine Bewertungen
- Activity C07: Boyle's Law: Pressure - Volume Relationship in Gases (Pressure Sensor)Dokument6 SeitenActivity C07: Boyle's Law: Pressure - Volume Relationship in Gases (Pressure Sensor)john234748Noch keine Bewertungen
- Illustrated Microsoft Office 365 and Word 2016 For Medical Professionals Loose Leaf Version 1st Edition Duffy Test BankDokument36 SeitenIllustrated Microsoft Office 365 and Word 2016 For Medical Professionals Loose Leaf Version 1st Edition Duffy Test Bankgenevievetruong9ajpr100% (29)
- Phet Gas Law Simulation 2010Dokument8 SeitenPhet Gas Law Simulation 2010api-262304087Noch keine Bewertungen
- Hysys - Inductive Method - StyreneDokument7 SeitenHysys - Inductive Method - Styrenejenny2409Noch keine Bewertungen
- Gas Investigation 2014Dokument4 SeitenGas Investigation 2014BystanderNoch keine Bewertungen
- L4 HYSYS Tutorial - PART IDokument25 SeitenL4 HYSYS Tutorial - PART IThilina GunawardhanaNoch keine Bewertungen
- Lab # 15Dokument13 SeitenLab # 15Kashaf TehreemNoch keine Bewertungen
- Learn Hvac Cost: A Costing & Planning Software For HVAC EngineersDokument44 SeitenLearn Hvac Cost: A Costing & Planning Software For HVAC EngineersFaquruddinNoch keine Bewertungen
- Charles LawDokument8 SeitenCharles LawasilahrafarNoch keine Bewertungen
- Exercise DP.1: Estimating Pure Component Properties in Hysys Workshop Report RequirementsDokument8 SeitenExercise DP.1: Estimating Pure Component Properties in Hysys Workshop Report RequirementsHafiz AzizNoch keine Bewertungen
- Miguel, John Ryan, D.G Lab Act 7Dokument3 SeitenMiguel, John Ryan, D.G Lab Act 7Simon DeleonNoch keine Bewertungen
- CAPDLab 3 Thermo 2 FlashModelDokument8 SeitenCAPDLab 3 Thermo 2 FlashModelAamir HusainNoch keine Bewertungen
- A4 - Teuku Hilman Revanda - 001201500038Dokument9 SeitenA4 - Teuku Hilman Revanda - 001201500038teuku revandaNoch keine Bewertungen
- Chemthink Gas Laws TutorialDokument4 SeitenChemthink Gas Laws TutorialtrinityNoch keine Bewertungen
- Lab No 2Dokument3 SeitenLab No 2Sohira QaziNoch keine Bewertungen
- Boyle's Law Online Simulated ExperimentDokument6 SeitenBoyle's Law Online Simulated ExperimentKugan GaneMalNoch keine Bewertungen
- Maths ProjectDokument15 SeitenMaths ProjectShivam GusainNoch keine Bewertungen
- 06 Boyles Law VernierDokument4 Seiten06 Boyles Law VernierGiovanni GuzmanNoch keine Bewertungen
- Gas Properties SimulationDokument3 SeitenGas Properties SimulationAmal ZahraNoch keine Bewertungen
- HYSYSTutorial CHEE332Dokument35 SeitenHYSYSTutorial CHEE332Syukri ShahNoch keine Bewertungen
- Mollier DiagramDokument17 SeitenMollier Diagrammister_no34Noch keine Bewertungen
- Boyle's Law: Pressure-Volume Relationship in Gases: ComputerDokument5 SeitenBoyle's Law: Pressure-Volume Relationship in Gases: Computerkaren liewNoch keine Bewertungen
- ReportDokument3 SeitenReportpp9p2m5x5gNoch keine Bewertungen
- Soda and Celsius: An Experiment With Heat and Temperature Using CarbonationDokument5 SeitenSoda and Celsius: An Experiment With Heat and Temperature Using CarbonationmaryjaneapuadaNoch keine Bewertungen
- q4 Las2 g10 Science Boyles-LawDokument9 Seitenq4 Las2 g10 Science Boyles-Lawtheobarrios14Noch keine Bewertungen
- HumidityDokument17 SeitenHumidityjokishNoch keine Bewertungen
- 5-1: Boyle's Law: Pressure and VolumeDokument2 Seiten5-1: Boyle's Law: Pressure and VolumeEmmanuel ChidiacNoch keine Bewertungen
- Student Exploration: Boyle's Law and Charles's LawDokument12 SeitenStudent Exploration: Boyle's Law and Charles's LawMF - 11AK 827776 Central Peel SSNoch keine Bewertungen
- Chemistry-I 1ST QTR PDFDokument2 SeitenChemistry-I 1ST QTR PDFAtharrizwanNoch keine Bewertungen
- Eligibility Conditions Qualification / Age Category of Selection Qualification AgeDokument4 SeitenEligibility Conditions Qualification / Age Category of Selection Qualification AgeAtharrizwanNoch keine Bewertungen
- PHY111 - Lesson 1Dokument21 SeitenPHY111 - Lesson 1AtharrizwanNoch keine Bewertungen
- DAANISH SCHOOL (BOYS / GIRLS) - : Application Form For Admission of Class 6 Entry For The YearDokument4 SeitenDAANISH SCHOOL (BOYS / GIRLS) - : Application Form For Admission of Class 6 Entry For The YearAtharrizwanNoch keine Bewertungen
- Magnetism in Transition Metal Complexes: Spin-Only Magnetic Moments ( ) Are Usually Observed For FirstDokument6 SeitenMagnetism in Transition Metal Complexes: Spin-Only Magnetic Moments ( ) Are Usually Observed For FirstAtharrizwanNoch keine Bewertungen
- Trig Project Part-1: in RedDokument5 SeitenTrig Project Part-1: in RedAtharrizwanNoch keine Bewertungen