Beruflich Dokumente
Kultur Dokumente
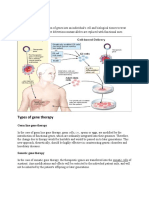
Gene Therapy
Hochgeladen von
Pramod Thapa0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
8 Ansichten2 Seitensurgery lecture: gene therapy
Copyright
© © All Rights Reserved
Verfügbare Formate
DOCX, PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldensurgery lecture: gene therapy
Copyright:
© All Rights Reserved
Verfügbare Formate
Als DOCX, PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
8 Ansichten2 SeitenGene Therapy
Hochgeladen von
Pramod Thapasurgery lecture: gene therapy
Copyright:
© All Rights Reserved
Verfügbare Formate
Als DOCX, PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 2
GENE THEREPY Multigene or multifactorial disorders such
as these would be especially difficult to
Short-lived nature of gene therapy – treat effectively using gene therapy
Before gene therapy can become a
permanent cure for any condition, the For countries in which germ-line gene
therapeutic DNA introduced into target therapy is illegal, indications that
cells must remain functional and the cells the Weismann barrier (between soma and
containing the therapeutic DNA must be germ-line) can be breached are relevant;
long-lived and stable. spread to the testes, therefore could
impact the germline against the intentions
Problems with integrating therapeutic DNA of the therapy.
into the genome and the rapidly dividing
nature of many cells prevent gene therapy Chance of inducing a tumor (insertional
from achieving any long-term benefits. mutagenesis) – If the DNA is integrated in
the wrong place in the genome, for
Patients will have to undergo multiple
example in a tumor suppressor gene, it
rounds of gene therapy.
could induce a tumor.
Immune response – Any time a foreign
This has occurred in clinical trials for X-
object is introduced into human tissues,
linked severe combined immunodeficiency
the immune system is stimulated to attack
(X-SCID) patients, in which hematopoietic
the invader.
stem cells were transduced with a
The risk of stimulating the immune system corrective transgene using a retrovirus,
in a way that reduces gene therapy and this led to the development of T cell
effectiveness is always a possibility. leukemia in 3 of 20 patients.
Furthermore, the immune system's One possible solution for this is to add a
enhanced response to invaders that it has functional tumor suppressor gene onto the
seen before makes it difficult for gene DNA to be integrated; however, this poses
therapy to be repeated in patients. its own problems, since the longer the
DNA is, the harder it is to integrate it
Problems with viral vectors – Viruses, the efficiently into cell genomes
carrier of choice in most gene therapy
studies, present a variety of potential The cost - only a small number of patients
problems to the patient: can be treated with gene therapy because
of the extremely high cost (Alipogene
toxicity, immune and inflammatory tiparvovec or Glybera, for example, at a
responses, and gene control and targeting cost of $1.6 million per patient was
issues. reported in 2013 to be the most expensive
drug in the world)
In addition, there is always the fear that
the viral vector, once inside the patient, 2014
may recover its ability to cause disease.
In January 2014, researchers at
Multigene disorders – Conditions or the University of Oxford reported that six
disorders that arise from mutations in a people suffering from choroideremia had
single gene are the best candidates for been treated with a genetically engineered
gene therapy. adeno-associated virus with a copy of a
gene REP1.
Unfortunately, some of the most
commonly occurring disorders, such Over a six month to two year
as heart disease, high blood period all had improved their
pressure, Alzheimer's disease, arthritis, sight. Choroideremia is an
and diabetes, are caused by the combined inherited genetic eye disease for
effects of variations in many genes. which in the past there has been
no treatment and patients
eventually go blind.
In March 2014 researchers at kinds of cells. Scientists can now
the University of Pennsylvania reported investigate what different genes do and
that 12 patients with HIV had been treated how they work together much more rapidly
since 2009 in a trial with a genetically and comprehensively.
engineered virus with a rare mutation
CRISPR relies on a programmable
known to protect against HIV
molecular machine (an enzyme) called
(CCR5 deficiency). Results were
Cas9 that binds to a small-guide RNA
promising
molecule. Together, these components
Sixty years ago, the question was: what home in on the target gene and carry out
does our genetic code look like? Then: precise molecular “surgery” to create a
how many genes make up our DNA? After genetic change. This can be used to
that: which genes cause diseases? correct a defect that causes a genetic
disease. The technology is young, and it
Now the question is: what if we could will take time to fully realize this promise.
repair broken genes? Some diseases will be more challenging
than others, and there is a lot of work to be
It has been a goal of doctors and scientists
done to further extend CRISPR’s
for decades to correct disease-causing
capabilities.
mistakes in our DNA. A new technology
called genome editing brings us closer to However, we are at a watershed moment
making that goal a reality. in our understanding of genomic science.
Not only have we identified many of the
Today we know that there are over 5,000
mutations that cause a variety of diseases
genes that cause genetic diseases, the
but we have also identified a technology
majority of which have no cure or
with the potential to create novel
treatments. These are the diseases that
medicines that directly target and correct
stand to benefit most from genomic
those mutations.
medicine and, specifically, the newest and
most powerful genome-editing technology This is just one of the many areas we are
called CRISPR (pronounced “crisper”). exploring at Editas. We believe we have
We hope that many severe and life- the opportunity to unlock a broad class of
threatening diseases can be treated by new transformative genomic medicines
technologies such as CRISPR: diseases that will enable precise, corrective
such as cystic fibrosis, which attacks the modifications to DNA to treat the
lungs and digestive system; Duchenne underlying causes of genetic diseases.
muscular dystrophy (DMD), a muscle More importantly, we’re getting closer to
disease; and sickle cell disease, a making once untreatable
debilitating blood disorder. conditions treatable by repairing broken
genes.
By “correcting” genetic defects in patients
with these diseases, we hope to restore
the normal function of the gene and
significantly improve quality of life. For
patients with DMD, this could mean being
able to walk and breathe better; for
patients with cystic fibrosis, this could
mean breathing more easily; and for
patients with sickle cell disease, this could
mean reducing the painful crises caused
by the disease.
CRISPR has taken the scientific research
community by storm because it is easy to
use and it can make DNA changes in
many different settings and many different
Das könnte Ihnen auch gefallen
- Genetic disorders and recent developments in gene therapyVon EverandGenetic disorders and recent developments in gene therapyNoch keine Bewertungen
- What Is Gene TherapyDokument5 SeitenWhat Is Gene TherapyRitwik BramhachariNoch keine Bewertungen
- What Is Gene TherapyDokument22 SeitenWhat Is Gene TherapyAbsar AlamNoch keine Bewertungen
- Gene TherapyDokument42 SeitenGene Therapyדודמתיו100% (1)
- 72 FullDokument2 Seiten72 FullAijaz AhmadNoch keine Bewertungen
- Gene TherapyDokument16 SeitenGene TherapyYhan Brotamonte BoneoNoch keine Bewertungen
- Gene Therapy 1Dokument25 SeitenGene Therapy 1Shobha RangappaNoch keine Bewertungen
- Gene Therapy - Arpita MishraDokument26 SeitenGene Therapy - Arpita MishraBiotech PressNoch keine Bewertungen
- Gene Therapy1Dokument26 SeitenGene Therapy1fangekeNoch keine Bewertungen
- Gene Therapy 1Dokument22 SeitenGene Therapy 1Priya ChaudharyNoch keine Bewertungen
- Gene Therapy - PresentationDokument19 SeitenGene Therapy - PresentationElectRon ShajalNoch keine Bewertungen
- Abstract-Gene Therapy Can Be Broadly Defined As The Transfer of Genetic Material To Cure ADokument4 SeitenAbstract-Gene Therapy Can Be Broadly Defined As The Transfer of Genetic Material To Cure ALucifuge RofocaleNoch keine Bewertungen
- 9' - Gene TherapyDokument8 Seiten9' - Gene TherapyALNAKI100% (1)
- gene therapyDokument7 Seitengene therapySaba RiazNoch keine Bewertungen
- Approach: Genes Cells Biological Tissues Disease MutatedDokument9 SeitenApproach: Genes Cells Biological Tissues Disease MutatedrajnikhatkarNoch keine Bewertungen
- Genetic CounselingDokument28 SeitenGenetic CounselingAbishaNoch keine Bewertungen
- Gene Therapy ExplainedDokument40 SeitenGene Therapy ExplainedMahbubul Islam Koushick100% (1)
- Gene TherapyDokument16 SeitenGene TherapyMargarita ArnoldNoch keine Bewertungen
- Define Gene Therapy.: 3. Explain The Ethical Considerations in Germ Line Gene TherapyDokument5 SeitenDefine Gene Therapy.: 3. Explain The Ethical Considerations in Germ Line Gene TherapyJake AlarconNoch keine Bewertungen
- Gene TherapyDokument7 SeitenGene Therapydarlene consignado100% (1)
- Gene TherapyDokument30 SeitenGene TherapypklarenzanneNoch keine Bewertungen
- Wa0001Dokument14 SeitenWa0001Hema ChandrikaNoch keine Bewertungen
- Gene Therapy1Dokument40 SeitenGene Therapy1Sindhu SmileyNoch keine Bewertungen
- Gene Therapy BiologyDokument26 SeitenGene Therapy BiologySagnik ChakrabortyNoch keine Bewertungen
- Human Gene TherapyDokument7 SeitenHuman Gene Therapymichelleannmaguigad28Noch keine Bewertungen
- GeneDokument56 SeitenGeneACTION plusNoch keine Bewertungen
- Biology - Kashvi - Gene TherapyDokument56 SeitenBiology - Kashvi - Gene TherapyKashvi ShriNoch keine Bewertungen
- Gene Therapy 1Dokument25 SeitenGene Therapy 1Casey MerencilloNoch keine Bewertungen
- The Aspects of Gene TherapyDokument7 SeitenThe Aspects of Gene TherapyKian Shane MedollarNoch keine Bewertungen
- Gene Delivery ThesisDokument5 SeitenGene Delivery ThesisHelpWritingPapersUK100% (2)
- Gene TherapyDokument19 SeitenGene TherapySmyle KatariaNoch keine Bewertungen
- Assignment No. 1: Gene TherapyDokument5 SeitenAssignment No. 1: Gene Therapykhadija tariqNoch keine Bewertungen
- Patient Treatments: Advances and ChallengesDokument81 SeitenPatient Treatments: Advances and Challengesyudha fahmiNoch keine Bewertungen
- Gene Therapy.Dokument74 SeitenGene Therapy.Mankaran Singh100% (2)
- Gene Therapy PPT by Mahi Bhardwaj (21 - 4693)Dokument21 SeitenGene Therapy PPT by Mahi Bhardwaj (21 - 4693)12pmtnl21000200.mahiNoch keine Bewertungen
- Introduction To Gene Therapy: 1.1 Genes As DrugsDokument7 SeitenIntroduction To Gene Therapy: 1.1 Genes As DrugsJason LingNoch keine Bewertungen
- Viral and Nonviral Delivery Systems For Gene Delivery: July 2012Dokument12 SeitenViral and Nonviral Delivery Systems For Gene Delivery: July 2012Arian JavidNoch keine Bewertungen
- Group 8 STS - Gene TheraphyDokument35 SeitenGroup 8 STS - Gene TheraphyArsenio RojoNoch keine Bewertungen
- Gene TherapyDokument18 SeitenGene TherapytennisondxbNoch keine Bewertungen
- Human Gene Therapy: Related PapersDokument8 SeitenHuman Gene Therapy: Related PapersLordsam B. ListonNoch keine Bewertungen
- Ukamaka SeminarDokument19 SeitenUkamaka SeminarMarycynthiaNoch keine Bewertungen
- Inder VermaDokument5 SeitenInder Vermaapi-353032101Noch keine Bewertungen
- Gene Therapy - Promises, Problems and ProspectsDokument4 SeitenGene Therapy - Promises, Problems and ProspectsLordsam B. ListonNoch keine Bewertungen
- GENE THERAPY: AN EXPERIMENTAL TECHNIQUE FOR TREATING DISEASEDokument20 SeitenGENE THERAPY: AN EXPERIMENTAL TECHNIQUE FOR TREATING DISEASEArlene Poso BalaNoch keine Bewertungen
- A Review On Gene Therapy: History, Vectors, Technologies and ApplicationDokument23 SeitenA Review On Gene Therapy: History, Vectors, Technologies and ApplicationBantuinAku KakNoch keine Bewertungen
- Biology ProjectDokument18 SeitenBiology Project39 Aditiya DuttaNoch keine Bewertungen
- Gene TherapyDokument2 SeitenGene TherapyVam UriarteNoch keine Bewertungen
- gene therapyDokument9 Seitengene therapySumaNoch keine Bewertungen
- Gene TherapyDokument3 SeitenGene TherapyGleeson Jay NiedoNoch keine Bewertungen
- Gene Therapy: Hereditary or Acquired Genetic DefectsDokument66 SeitenGene Therapy: Hereditary or Acquired Genetic DefectsDRx Sonali TareiNoch keine Bewertungen
- Gene TherapyDokument8 SeitenGene TherapyAnjali AnilNoch keine Bewertungen
- L - 10 Gene TherapyDokument6 SeitenL - 10 Gene TherapyDimple CobachaNoch keine Bewertungen
- Gene TherapiesDokument15 SeitenGene Therapiesenfanat23Noch keine Bewertungen
- Gene Therapy 2Dokument37 SeitenGene Therapy 2Snegapriya SivaramanNoch keine Bewertungen
- Advances in Immunology: Genetic EngineeringDokument25 SeitenAdvances in Immunology: Genetic EngineeringEdelainne Joyce MontibonNoch keine Bewertungen
- Mechanisms Involved in Gene Therapy An OverviewDokument2 SeitenMechanisms Involved in Gene Therapy An OverviewKhaled M FawzyNoch keine Bewertungen
- Gene TheraphyDokument73 SeitenGene TheraphyPauleen Castillo Costiniano100% (1)
- Gene Theraphy Module14Dokument24 SeitenGene Theraphy Module14Anime HubNoch keine Bewertungen
- Immune Response in Cervical Intraepithelial Neoplasms: 10.31083/j.ejgo4205146Dokument9 SeitenImmune Response in Cervical Intraepithelial Neoplasms: 10.31083/j.ejgo4205146Liliana Antonio RevueltaNoch keine Bewertungen
- Human Gene Therapy: A Brief Overview of The Genetic RevolutionDokument7 SeitenHuman Gene Therapy: A Brief Overview of The Genetic RevolutionDinda Wanodya SNoch keine Bewertungen
- Cardiac DrugsDokument10 SeitenCardiac Drugssurviving nursing school100% (3)
- Cardiac DrugsDokument10 SeitenCardiac Drugssurviving nursing school100% (3)
- DENGUEDokument19 SeitenDENGUEPramod ThapaNoch keine Bewertungen
- PFCMAtrix ToolkitDokument7 SeitenPFCMAtrix ToolkitRold Brio Sos100% (3)
- Impact of HIV/AIDS On An Overseas Filipino Worker and His FamilyDokument6 SeitenImpact of HIV/AIDS On An Overseas Filipino Worker and His FamilyPramod ThapaNoch keine Bewertungen
- Neurology LocalizationDokument6 SeitenNeurology LocalizationPramod ThapaNoch keine Bewertungen
- 4 GeneCraftDokument10 Seiten4 GeneCraftRey JannahNoch keine Bewertungen
- Cell Science at a Glance: The JAK/STAT Signaling PathwayDokument3 SeitenCell Science at a Glance: The JAK/STAT Signaling Pathwayvijaykumar_vinniNoch keine Bewertungen
- Caractere VirologiaueDokument29 SeitenCaractere Virologiauehind el hamriNoch keine Bewertungen
- Molecular Biology of The Cell 5th Edition Alberts Test BankDokument13 SeitenMolecular Biology of The Cell 5th Edition Alberts Test Bankodettedieupmx23m100% (29)
- Biology Folio Form 5 VariationDokument12 SeitenBiology Folio Form 5 VariationSentoash NaiduNoch keine Bewertungen
- Target Amplification MethodsDokument3 SeitenTarget Amplification MethodsNOR-FATIMAH BARATNoch keine Bewertungen
- 12 Biology Genetics Exam Practise With AnswersDokument8 Seiten12 Biology Genetics Exam Practise With AnswersBrooke GoodingNoch keine Bewertungen
- Jurnal Internasional Isolasi DNADokument6 SeitenJurnal Internasional Isolasi DNAGregorius SimbolonNoch keine Bewertungen
- Topical Past Yr Essay QuestionsDokument8 SeitenTopical Past Yr Essay QuestionsThuran Nathan100% (1)
- Ich GuidelinesDokument9 SeitenIch GuidelinessamNoch keine Bewertungen
- List of Industries Booking Effluent Under 20 MLD in Ankleshwar and PanoliDokument2 SeitenList of Industries Booking Effluent Under 20 MLD in Ankleshwar and PanoliSupriyaNoch keine Bewertungen
- Genentech: The Beginnings of Biotech by Sally Smith HughesDokument2 SeitenGenentech: The Beginnings of Biotech by Sally Smith HughesRicha Malhotra0% (1)
- Chapter 23 BacteriaDokument6 SeitenChapter 23 BacteriaBlairNoch keine Bewertungen
- Mark Ptashne and Alexander Gann - Genes and Signals (2001)Dokument209 SeitenMark Ptashne and Alexander Gann - Genes and Signals (2001)hassan haddadiNoch keine Bewertungen
- Nyctotherus Ovalis and Its RelativesDokument12 SeitenNyctotherus Ovalis and Its RelativesSandy VasquezNoch keine Bewertungen
- Dr. Magdalena C. Cantoria Accomplished BotanistDokument2 SeitenDr. Magdalena C. Cantoria Accomplished BotanistLynzee Reyes100% (1)
- Tos - Final - Exam Earth and Life Science 1Dokument2 SeitenTos - Final - Exam Earth and Life Science 1Rudula Amper100% (1)
- SCOPE OF CLINICAL PHARMACYDokument13 SeitenSCOPE OF CLINICAL PHARMACYkeerthanaNoch keine Bewertungen
- List of Cro in IndiaDokument3 SeitenList of Cro in Indiajaykardani_20% (1)
- Infectious AIDS, Have We Been Misled?Dokument590 SeitenInfectious AIDS, Have We Been Misled?ConventionalThinker100% (3)
- Are Viruses Living or Non-Living Organisms?Dokument8 SeitenAre Viruses Living or Non-Living Organisms?Sydney Cloyce NagalNoch keine Bewertungen
- SABRAO J Breed Genet 50 2 129 144 HARSONO PDFDokument16 SeitenSABRAO J Breed Genet 50 2 129 144 HARSONO PDFTRI HARSONONoch keine Bewertungen
- DNA ProfilingDokument4 SeitenDNA ProfilingVictorija DeldioNoch keine Bewertungen
- Meiosis: Chromatids (The Two Halves of A Duplicated Chromosome), As inDokument29 SeitenMeiosis: Chromatids (The Two Halves of A Duplicated Chromosome), As inyamamaNoch keine Bewertungen
- DNA replication stepsDokument3 SeitenDNA replication stepsKimNoch keine Bewertungen
- Conference On Rnai Based Pesticides ProgrammeDokument7 SeitenConference On Rnai Based Pesticides ProgrammeThornoo1Noch keine Bewertungen
- Six Month Safety and Efficacy of The BNT162b2 mRNA COVID-19 Vaccine - MedrxivDokument1 SeiteSix Month Safety and Efficacy of The BNT162b2 mRNA COVID-19 Vaccine - MedrxivKristina MilićevićNoch keine Bewertungen
- Devi Ahilya Vishwavidyalaya, Indore: School of PharmacyDokument7 SeitenDevi Ahilya Vishwavidyalaya, Indore: School of PharmacyKrishnakant KushwahaNoch keine Bewertungen
- (Svante Pääbo Et Al) Genetic Analyses From Ancient DNA PDFDokument35 Seiten(Svante Pääbo Et Al) Genetic Analyses From Ancient DNA PDFPhilipe AfonsoNoch keine Bewertungen
- 3 CancerDokument47 Seiten3 Cancerkirubel getyeNoch keine Bewertungen
- LIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionVon EverandLIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionBewertung: 4 von 5 Sternen4/5 (402)
- Why We Die: The New Science of Aging and the Quest for ImmortalityVon EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityBewertung: 3.5 von 5 Sternen3.5/5 (2)
- Outlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisVon EverandOutlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisBewertung: 4 von 5 Sternen4/5 (1)
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedVon EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedBewertung: 5 von 5 Sternen5/5 (78)
- The Age of Magical Overthinking: Notes on Modern IrrationalityVon EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityBewertung: 4 von 5 Sternen4/5 (13)
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeVon EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeNoch keine Bewertungen
- The Comfort of Crows: A Backyard YearVon EverandThe Comfort of Crows: A Backyard YearBewertung: 4.5 von 5 Sternen4.5/5 (23)
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsVon EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsBewertung: 3.5 von 5 Sternen3.5/5 (3)
- Techniques Exercises And Tricks For Memory ImprovementVon EverandTechniques Exercises And Tricks For Memory ImprovementBewertung: 4.5 von 5 Sternen4.5/5 (40)
- Raising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsVon EverandRaising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsBewertung: 5 von 5 Sternen5/5 (1)
- Raising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsVon EverandRaising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsBewertung: 4.5 von 5 Sternen4.5/5 (169)
- The Obesity Code: Unlocking the Secrets of Weight LossVon EverandThe Obesity Code: Unlocking the Secrets of Weight LossBewertung: 5 von 5 Sternen5/5 (4)
- The Ultimate Guide To Memory Improvement TechniquesVon EverandThe Ultimate Guide To Memory Improvement TechniquesBewertung: 5 von 5 Sternen5/5 (34)
- Roxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingVon EverandRoxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingBewertung: 5 von 5 Sternen5/5 (5)
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaVon EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- By the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsVon EverandBy the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsNoch keine Bewertungen
- Summary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisVon EverandSummary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisBewertung: 5 von 5 Sternen5/5 (8)
- Roxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingVon EverandRoxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingBewertung: 3.5 von 5 Sternen3.5/5 (33)
- Dark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Von EverandDark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Bewertung: 4.5 von 5 Sternen4.5/5 (110)
- Summary: It Didn't Start with You: How Inherited Family Trauma Shapes Who We Are and How to End the Cycle By Mark Wolynn: Key Takeaways, Summary & AnalysisVon EverandSummary: It Didn't Start with You: How Inherited Family Trauma Shapes Who We Are and How to End the Cycle By Mark Wolynn: Key Takeaways, Summary & AnalysisBewertung: 5 von 5 Sternen5/5 (3)
- The Happiness Trap: How to Stop Struggling and Start LivingVon EverandThe Happiness Trap: How to Stop Struggling and Start LivingBewertung: 4 von 5 Sternen4/5 (1)
- The Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeVon EverandThe Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeBewertung: 4.5 von 5 Sternen4.5/5 (253)
- The Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsVon EverandThe Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsNoch keine Bewertungen
- The Tennis Partner: A Doctor's Story of Friendship and LossVon EverandThe Tennis Partner: A Doctor's Story of Friendship and LossBewertung: 4.5 von 5 Sternen4.5/5 (4)