Beruflich Dokumente
Kultur Dokumente
General Chemistry: CHEM F111
Hochgeladen von
Harsh Tiwari0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
22 Ansichten29 SeitenOriginaltitel
GenChem lecture 8
Copyright
© © All Rights Reserved
Verfügbare Formate
PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
22 Ansichten29 SeitenGeneral Chemistry: CHEM F111
Hochgeladen von
Harsh TiwariCopyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 29
BITS Pilani
Pilani Campus
CHEM F111 General Chemistry Lecture 08
Hydrogenic atom: A quantum
mechanical treatment (continued)
Review of lecture 07
• Central force field and spherical polar coordinates
• Schrӧdinger equation for Hydrogenic atom
• Radial wavefunctions and spherical harmonics
• Quantum numbers, quantization in energy and
magnitude and orientation of angular momentum
• Shells, subshells, orbitals
• Orbital characterization, Radial and angular nodes
• Form of the wavefunctions with for ml=0 and ml≠ 0
BITSPilani, Pilani Campus
Hydrogenic orbitals (revision)
The wavefunctions for the hydrogenic atom, in
spherical polar coordinates, may be shown to factor as
ψ(r, θ, φ) = R(r)Θ(θ)Φ(φ)
Further, the requirement that the wavefunction be
well-behaved, (i.e., single valued, continuous,..) leads
to the result that the functions are labeled by three
quantum numbers n, l, and ml ie.,
ψn,l,ml(r,θ,φ) = Rn,l(r)Θl,ml(θ)Φml(φ) = Rn,l(r)Yl,ml(θ, φ)
where Rn,l(r) is called the radial part of ψ (radial
wavefunction), and Yl,ml(θ, φ) is its angular part – the
spherical harmonics.
BITSPilani, Pilani Campus
Shells, subshells and orbitals
(revision)
What values can the quantum numbers take ?
• n = 1, 2, 3,…..
Shell is determined by n (characterized by energy)
• l = 0, 1, 2,.., n-1, for given value of n
• For a given n, there are n subshells. Each subshell is
characterized by the radial wavefunction, Rn,l(r); l
=0,1,2,3,4,5,... corresponds to s, p, d, f, g, h,....
subshells of a given shell.
• For given l, there are 2l + 1 orbitals corresponding to
ml = -l, -l+1,..,0,..l-1, l.
• How many orbitals for a given n? → n2
BITSPilani, Pilani Campus
Hydrogenic orbitals-
characteristics
The general form is ψn,l,ml(r,θ,φ) = Rn,l(r)Yl,ml(θ, φ)
Number of radial nodes = degree of polynomial in r
Azimuthal quantum number Principle quantum
equals to no of angular nodes number
Yl,m (θ, φ) are spherical harmonics: Polynomial of degree,
l
“l” in cosθ and/or sinθ multiplied by Φml(φ)
BITSPilani, Pilani Campus
Orbitals with ml=0 (revision)
Orbitals with ml=0, l=0
Orbitals with ml=0, l=1
BITSPilani, Pilani Campus
Orbitals with ml=0 (revision)
Orbitals with ml=0, l=2,3
BITSPilani, Pilani Campus
Orbitals with non-zero z-component
of orbital angular momentum
These orbitals are characterized by |ml|>0. If one probes to
measure the exact value of z-component of the angular
momentum in addition to its magnitude, the orbitals are
complex with Φm (φ) = eimlφ
l
For example,
Note: In all the orbital expressions, M is normalization constant
and is different for different orbitals.
BITSPilani, Pilani Campus
Orbitals with non-zero z-component
of orbital angular momentum
However, the behavior with respect to x and y directions
cannot be determined for these orbitals.
Alternately, one can choose the set obtained by linear
combination of orbital-pairs of given {l, |ml|}; and the
behavior with respect to x and y directions can be
studied at the cost of uncertainty in ml.
Recall the degenerate and non-degenerate eigenfunctions of
operators discussed in lecture 03.
BITSPilani, Pilani Campus
Linear combinations of p+1 and p-1
yields px and py orbitals.
BITSPilani, Pilani Campus
Linear combinations of d+1 and d-1
yields dxz and dyz orbitals.
BITSPilani, Pilani Campus
Linear combinations of d+2 and d-2
yields dx2-y2 and dxy orbitals.
BITSPilani, Pilani Campus
Orthogonal functions
The functions {fk(τ)} are said to be mutually
orthogonal if
∫fi* fjdτ =0 if i≠j; and ∫fi* fjdτ ≠0 if i=j
(^^^ apropriate limits of integration to be used)
Functions which are normalized and mutually
orthogonal are said to be orthonormal.
It can be verified that the hydrogenic orbitals are all
mutually orthogonal. (Try it yourself for a few orbitals).
BITSPilani, Pilani Campus
Orthogonal functions
We will now prove that the Hamiltonian eigenfunctions for
particle in 1-D box are all mutually orthogonal.
L nπ x mπx
(2/ L)∫0 sin ( )sin( )dx ; (n≠m)
L L
2 1 L πx πx
=( ) ∫0 {cos[(n−m) ]−cos [(n+ m) ]}dx
L 2 L L
L
(n−m)π x ( n+m)π x
( )
sin [ ] sin [ ]
1 L L
= − =0
L (n−m) π (n+ m) π
L L 0
Homework: Show that the Hamiltonian eigenfunctions
for 1-D harmonic oscillator corresponding to the states:
v=0 and v=1 are orthogonal.
Is this observation just a coincidence or any law governs
it?
BITSPilani, Pilani Campus
Hermitian operators (FYI)
●
Hermitian operator is the one, which satisfies a particular
mathematical relation, which ensures that eigenvalues of the
operator cannot be complex or imaginary. (We won't worry
about the math. Just the fact, that, eigenvalues of hermitian
operators are real, is important to us).
●
The operators corresponding to physical observables (e.g. K.E.,
P.E., Hamitonian, Angular momentum, linear momentum,
position, etc ) are all hermitian.
●
Non-degenerate eigenfunctions of hermitian operator are
always mutually orthogonal. Degenerate eigenfunctions can be
chosen to be orthogonal.
BITSPilani, Pilani Campus
Operators act as probes for the
corresponding observables (FYI)
The Schrödinger equation for hydrogenic atom written
is spherical coordinates, probes the orbital angular
momentum and its z-component due to which one can
obtain exact magnitude of angular momentum and
also its z-component, in addition to the energy.
If we do not probe the measurement of angular
momentum or its z-component, all that we can get is
the energy corresponding to quantum number n, and
the information that it is n2-fold degenerate.
BITSPilani, Pilani Campus
Operators act as probes for the
corresponding observables (FYI)
Question: Is it necessary that the hydrogenic atom
must exist in a pure-angular momentum state (that is,
a state characterized by a definite azimuthal quantum
number, l)?
The answer is, NO, unless we probe to measure the
angular momentum of the system.
Since, the orbitals corresponding to same value of
principle quantum number n, are degenerate
(eigenvalue: E n) wrt Hamiltonian, any linear
combination of the orbitals of a given shell would also
be eigenfunction of the Hamiltonian with same
eigenvalue En.
BITSPilani, Pilani Campus
Operators act as probes for the
corresponding observables (FYI)
From n2 orbitals (differing in l and/or ml), one can
obtain orthogonal set of n2 orbitals – hybrid orbitals:
e.g. Consider an orbital: Ψhybrid = c.ψ2s + (1-c2)1/2ψ2pz is a
hybrid orbital with n=2. The coefficients c and (1-c2)1/2
result from the orthonormality of the pure angular
momentum orbitals (2s, 2pz).
If the ratio, c2:(1-c2) = 1:n, the Ψhybrid is said to be spn
hybrid – To be discussed in detail later, in the context
of chemical bonding
BITSPilani, Pilani Campus
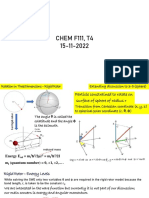
Energy levels of hydrogenic
atoms
BITSPilani, Pilani Campus
Ground state of hydrogenic
atom
Ground State: n = 1, l = 0, ml = 0 → 1s orbital
Ψ1,0,0 = (4π-1/2)(4/a03)1/2 e-r/a0 (independent of θ and φ →
true for all s states since Y0,0 = (4π-1/2).
All s orbitals are spherically symmetric, related to their
being states of zero angular momentum.
Probability density depends only on r; it is maximum at
r=0 (nucleus) and decays exponentially with distance r.
No nodes
BITSPilani, Pilani Campus
1s wavefunction and probability
density
BITSPilani, Pilani Campus
Radial probability distribution
Radial probability distribution function: Probability that
electron will be found between r and r+dr from the
nucleus, regardless of the angle/direction. For s
orbitals,
P(r)dr = 4πr2R2dr (for orbitals with l=0, i.e., s-orbitals)
More generally P(r) = r2R2(r); (for orbitals with l>0)
where R(r) is the radial wavefunction
For the 1s orbital (that is, for the ground state),
P(r) = (4/a03) 4πr2 e-2r/a0
BITSPilani, Pilani Campus
Radial probability distribution
for 1s state
Most probable distance from the nucleus = a0
How to obtain this? Set dP(r)/dr = 0 and calculate r
BITSPilani, Pilani Campus
Radial wavefunctions
The general form of the radial part of the
hydrogenic atom wavefunctions, obtained by
solving the radial Schrodinger equation, and
applying the necessary conditions on the solutions,
may be written
l -Zr/na0
•
Rn,l (r) α r . (Polynomial of degree n-l-1). e
•The first factor determines the behaviour at r = 0
(only l = 0 wavefunctions are nonzero at the origin)
•The second factor determines the number of radial
nodes (n-l-1)
• The third ensures that the function goes to 0 as r→∞
BITSPilani, Pilani Campus
Radial wavefunctions
BITSPilani, Pilani Campus
1s, 2s and 3s orbitals
Spherically symmetric, angular part is just a
constant
States of zero orbital angular momentum
1s
2s
3s
BITSPilani, Pilani Campus
p orbitals angular parts
α cos θ (pz) α sin θ sin φ (py) α sin θ cos φ (px)
l = 1, ml = 0 l = 1, ml = 1 or –1 l = 1, ml = 1 or –1
1 nodal surface 1 nodal surface (xz) 1 nodal surface (yz)
(xy)
BITSPilani, Pilani Campus
p orbitals boundary surfaces
BITSPilani, Pilani Campus
d orbitals angular parts
2 nodal planes
BITSPilani, Pilani Campus
Das könnte Ihnen auch gefallen
- GCL 08Dokument19 SeitenGCL 08sahanishubham317Noch keine Bewertungen
- Difference Equations in Normed Spaces: Stability and OscillationsVon EverandDifference Equations in Normed Spaces: Stability and OscillationsNoch keine Bewertungen
- Radial and Angular Parts of Atomic OrbitalsDokument7 SeitenRadial and Angular Parts of Atomic OrbitalsArchisman MisraNoch keine Bewertungen
- General Chemistry: CHEM F111Dokument30 SeitenGeneral Chemistry: CHEM F111Harsh TiwariNoch keine Bewertungen
- Radial and Angular Parts of Atomic OrbitalsDokument3 SeitenRadial and Angular Parts of Atomic OrbitalsW47K3R 27886Noch keine Bewertungen
- Operador Intercambio Exclusion de PauliDokument4 SeitenOperador Intercambio Exclusion de PauliSoraya BustamanteNoch keine Bewertungen
- Tight-Binding Model of Electronic StructuresDokument9 SeitenTight-Binding Model of Electronic StructuresJulian David Henao EscobarNoch keine Bewertungen
- QM2 HM2Dokument2 SeitenQM2 HM2jog1Noch keine Bewertungen
- General Chemistry: CHEM F111Dokument22 SeitenGeneral Chemistry: CHEM F111Harsh TiwariNoch keine Bewertungen
- Physics of Exchange Interactions: Tomasz DietlDokument74 SeitenPhysics of Exchange Interactions: Tomasz DietlJohn AllenNoch keine Bewertungen
- ANGULAR MOMENTUMDokument6 SeitenANGULAR MOMENTUMJuliana SonodaNoch keine Bewertungen
- Lecture 12Dokument14 SeitenLecture 12r prathapNoch keine Bewertungen
- Hydrogenic OrbitalsDokument6 SeitenHydrogenic OrbitalsRohan RaoNoch keine Bewertungen
- John Atwell Moody - Standing Waves (2015)Dokument288 SeitenJohn Atwell Moody - Standing Waves (2015)davedamro5Noch keine Bewertungen
- Indian Institute of Technology, Guwahati: CH101 Class 11 Physical ChemistryDokument5 SeitenIndian Institute of Technology, Guwahati: CH101 Class 11 Physical ChemistryMihir Kumar MechNoch keine Bewertungen
- Hamiltonian Parity BasicsDokument7 SeitenHamiltonian Parity BasicsRose AleNoch keine Bewertungen
- Convergent Inversion Approximations For Polynomials in Bernstein FormDokument18 SeitenConvergent Inversion Approximations For Polynomials in Bernstein FormMaiah DinglasanNoch keine Bewertungen
- Light Cone ReviewDokument55 SeitenLight Cone ReviewArrigo de FlandresNoch keine Bewertungen
- Wyklad ENG 2Dokument26 SeitenWyklad ENG 2sonphmNoch keine Bewertungen
- Atomic SpectrosDokument36 SeitenAtomic SpectrosAswin AlexNoch keine Bewertungen
- Quantum Mechanics Exam Solutions FYSN17/FMFN01Dokument4 SeitenQuantum Mechanics Exam Solutions FYSN17/FMFN01lolNoch keine Bewertungen
- GCL 06Dokument26 SeitenGCL 06sahanishubham317Noch keine Bewertungen
- Toeplitz Operators With Quasi-Separately Radial SymbolsDokument15 SeitenToeplitz Operators With Quasi-Separately Radial SymbolsJulio Cesar Jaramillo SandovalNoch keine Bewertungen
- BITS Pilani Chemistry Hamilton OperatorDokument11 SeitenBITS Pilani Chemistry Hamilton OperatorNaresh SehdevNoch keine Bewertungen
- AngmomDokument29 SeitenAngmomYılmaz ÇolakNoch keine Bewertungen
- Lecture 3Dokument5 SeitenLecture 3Anurag SNoch keine Bewertungen
- Classification of Rotacional Solitons in Warped ProductsDokument19 SeitenClassification of Rotacional Solitons in Warped ProductselismardbNoch keine Bewertungen
- Identical ParticlesDokument11 SeitenIdentical ParticlesShams ShamsNoch keine Bewertungen
- Orbital Angular MomentumDokument4 SeitenOrbital Angular MomentumjasmonNoch keine Bewertungen
- Journal of Algebra: Vesselin Drensky, Jen o Szigeti, Leon Van WykDokument10 SeitenJournal of Algebra: Vesselin Drensky, Jen o Szigeti, Leon Van WykLuis FuentesNoch keine Bewertungen
- Exam ED Aug SolDokument13 SeitenExam ED Aug SolAna SultanNoch keine Bewertungen
- Notes On Many Body Theory of Bose and Fermi Gases at Low TemperaturesDokument47 SeitenNotes On Many Body Theory of Bose and Fermi Gases at Low TemperaturesKami RaionNoch keine Bewertungen
- 10 Self-consistent field theory: ² ψ − ∇ ψ dr - ψ - r − r - ψ δ dr ψ - r − r - ψDokument4 Seiten10 Self-consistent field theory: ² ψ − ∇ ψ dr - ψ - r − r - ψ δ dr ψ - r − r - ψursml12Noch keine Bewertungen
- CHEMF111 Lecture5 Aug12 2016 - BPHCDokument23 SeitenCHEMF111 Lecture5 Aug12 2016 - BPHCAshish GuptaNoch keine Bewertungen
- Degeneracy of Energy Levels of Pseudo-Gaussian Oscillators: Articles You May Be Interested inDokument6 SeitenDegeneracy of Energy Levels of Pseudo-Gaussian Oscillators: Articles You May Be Interested inMohammedBujairNoch keine Bewertungen
- Relations Between Transition Rates and Quantum Numbers in Gravitational Potentials Plus FiguresDokument9 SeitenRelations Between Transition Rates and Quantum Numbers in Gravitational Potentials Plus FiguresaernestNoch keine Bewertungen
- Associated Legendre Functions and Dipole Transition Matrix ElementsDokument16 SeitenAssociated Legendre Functions and Dipole Transition Matrix ElementsFrancisco QuiroaNoch keine Bewertungen
- Lecture 10Dokument49 SeitenLecture 10Daniele PasseroneNoch keine Bewertungen
- Quantum Mechanics of Central PotentialsDokument7 SeitenQuantum Mechanics of Central PotentialsAjay KaladharanNoch keine Bewertungen
- The Schrödinger Wave Equation For The Hydrogen AtomDokument4 SeitenThe Schrödinger Wave Equation For The Hydrogen AtomDannie A. San PedroNoch keine Bewertungen
- Central PotDokument8 SeitenCentral PotSoumyadeep DasNoch keine Bewertungen
- Schroedinger Equation Atomic Wave Functions Atomic Orbitals Quantum NumbersDokument45 SeitenSchroedinger Equation Atomic Wave Functions Atomic Orbitals Quantum NumbersRishit JakhariaNoch keine Bewertungen
- Lec 7Dokument5 SeitenLec 7prakash mishraNoch keine Bewertungen
- QFT KleinGordonDokument71 SeitenQFT KleinGordonbob n sauveNoch keine Bewertungen
- Matrix Alg Slides DDokument44 SeitenMatrix Alg Slides DBill Erick CastilloNoch keine Bewertungen
- The Structure of AS-Gorenstein Algebras (Minamoto)Dokument35 SeitenThe Structure of AS-Gorenstein Algebras (Minamoto)navigetor23Noch keine Bewertungen
- Linear Algebra and its Applications: Functions of a matrix and Krylov matrices聻Dokument16 SeitenLinear Algebra and its Applications: Functions of a matrix and Krylov matrices聻krrrNoch keine Bewertungen
- Theoretische Physik 2: ElektrodynamikDokument8 SeitenTheoretische Physik 2: ElektrodynamikJuan P HDNoch keine Bewertungen
- Chapter 8: Orbital Angular Momentum And: Molecular RotationsDokument23 SeitenChapter 8: Orbital Angular Momentum And: Molecular RotationstomasstolkerNoch keine Bewertungen
- mf4 PDFDokument3 Seitenmf4 PDFAnonymous UrVkcd0% (1)
- Derivation of Plank Einstain ConstantDokument7 SeitenDerivation of Plank Einstain ConstantVidyesh KrishnanNoch keine Bewertungen
- Aqm - Rotations in Quantum Mechanics PDFDokument4 SeitenAqm - Rotations in Quantum Mechanics PDFTudor PatuleanuNoch keine Bewertungen
- 2 2 2 fi fi f f f −i - ω - ˆ n· ~ X i i i 2: Physics 6210/Spring 2007/Lecture 37Dokument6 Seiten2 2 2 fi fi f f f −i - ω - ˆ n· ~ X i i i 2: Physics 6210/Spring 2007/Lecture 37aslimanNoch keine Bewertungen
- Fermi Liquid NotesDokument14 SeitenFermi Liquid Notessakurai137Noch keine Bewertungen
- Answers by Mayank v20Dokument8 SeitenAnswers by Mayank v20GurujiNoch keine Bewertungen
- Parity and Charge Conjugation in Particle PhysicsDokument13 SeitenParity and Charge Conjugation in Particle PhysicsCalvin GaoNoch keine Bewertungen
- JT 205:quantum Mechanics Assignement 3: Due: 16 of December 2021 Answer All QuestionsDokument3 SeitenJT 205:quantum Mechanics Assignement 3: Due: 16 of December 2021 Answer All QuestionsMaitraNoch keine Bewertungen
- CHEM F111: General Chemistry Semester II: AY 2021-22: Lecture-03, 13 May 2022, FridayDokument25 SeitenCHEM F111: General Chemistry Semester II: AY 2021-22: Lecture-03, 13 May 2022, FridayROMIT RUNWALNoch keine Bewertungen
- The Tight Binding Method: 1 Background: A Hierarchy of MethodsDokument15 SeitenThe Tight Binding Method: 1 Background: A Hierarchy of MethodsSauvik ChatterjeeNoch keine Bewertungen
- Quora - How Was The Soviet Union Affected by The Great DepressionDokument7 SeitenQuora - How Was The Soviet Union Affected by The Great DepressionHarsh TiwariNoch keine Bewertungen
- The Story of Our Planet: How the Earth FormedDokument1 SeiteThe Story of Our Planet: How the Earth FormedHarsh TiwariNoch keine Bewertungen
- Quora - Why Was The USSR The Only World Power Whose Economy Thrived During The Great Depression, 1929-1933Dokument15 SeitenQuora - Why Was The USSR The Only World Power Whose Economy Thrived During The Great Depression, 1929-1933Harsh TiwariNoch keine Bewertungen
- Soviet Economy Thrived During Great DepressionDokument3 SeitenSoviet Economy Thrived During Great DepressionHarsh TiwariNoch keine Bewertungen
- Computer: Computer Is A Calculeting MachineDokument1 SeiteComputer: Computer Is A Calculeting MachineHarsh TiwariNoch keine Bewertungen
- The Black AeroplaneDokument1 SeiteThe Black AeroplaneHarsh TiwariNoch keine Bewertungen
- Quora - Why Wasn't The Soviet Union Greatly Affected During The Great Depression PDFDokument9 SeitenQuora - Why Wasn't The Soviet Union Greatly Affected During The Great Depression PDFHarsh TiwariNoch keine Bewertungen
- Quora - What Was The Result of The Great Depression On Russia's GovernmentDokument2 SeitenQuora - What Was The Result of The Great Depression On Russia's GovernmentHarsh TiwariNoch keine Bewertungen
- APA Book CitationDokument2 SeitenAPA Book CitationHarsh TiwariNoch keine Bewertungen
- TOPICDokument3 SeitenTOPICHarsh TiwariNoch keine Bewertungen
- (English) End of Space - Creating A Prison For Humanity (DownSub - Com)Dokument9 Seiten(English) End of Space - Creating A Prison For Humanity (DownSub - Com)Harsh TiwariNoch keine Bewertungen
- Blueprint of River Sand Mining Project in Yamuna River, District - Saharanpur, UpDokument1 SeiteBlueprint of River Sand Mining Project in Yamuna River, District - Saharanpur, UpHarsh TiwariNoch keine Bewertungen
- Legal and Economics Implications of Orbital Debris RemovalDokument8 SeitenLegal and Economics Implications of Orbital Debris RemovalSpace Frontier FoundationNoch keine Bewertungen
- GPS Base Space Debris Removal SystemDokument23 SeitenGPS Base Space Debris Removal SystemPruthvi ReddyNoch keine Bewertungen
- ReenaDokument2 SeitenReenaHarsh TiwariNoch keine Bewertungen
- PRATIKDokument1 SeitePRATIKHarsh TiwariNoch keine Bewertungen
- Harsh Mani Tiwari's Resume - B.E. (Hons.), Civil, 2021Dokument1 SeiteHarsh Mani Tiwari's Resume - B.E. (Hons.), Civil, 2021Harsh TiwariNoch keine Bewertungen
- Pratik - RaiDokument1 SeitePratik - RaiHarsh TiwariNoch keine Bewertungen
- Evaluation Scheme PDFDokument1 SeiteEvaluation Scheme PDFHarsh TiwariNoch keine Bewertungen
- Information and Communications Technology or Information and Communication TechnologyDokument3 SeitenInformation and Communications Technology or Information and Communication TechnologySimranjeet SinghNoch keine Bewertungen
- Ese Weightage PDFDokument1 SeiteEse Weightage PDFHarsh TiwariNoch keine Bewertungen
- 21st Century Skills: Writing: Paper SubmissionDokument1 Seite21st Century Skills: Writing: Paper SubmissionHarsh TiwariNoch keine Bewertungen
- Optional Subjects: ChemistryDokument6 SeitenOptional Subjects: ChemistryHarsh TiwariNoch keine Bewertungen
- Information and Communications Technology PDFDokument6 SeitenInformation and Communications Technology PDFHarsh TiwariNoch keine Bewertungen
- ICT AbstractDokument1 SeiteICT AbstractHarsh TiwariNoch keine Bewertungen
- 79425244Dokument120 Seiten79425244Harsh TiwariNoch keine Bewertungen
- David Crystal Interview: Why the Idea of a Standard English is a MythDokument17 SeitenDavid Crystal Interview: Why the Idea of a Standard English is a MythHarsh Tiwari100% (1)
- (English) End of Space - Creating A Prison For Humanity (DownSub - Com)Dokument9 Seiten(English) End of Space - Creating A Prison For Humanity (DownSub - Com)Harsh TiwariNoch keine Bewertungen
- Chapter 1Dokument26 SeitenChapter 1Harsh TiwariNoch keine Bewertungen
- APJ KalamDokument4 SeitenAPJ KalamHarsh TiwariNoch keine Bewertungen
- Bohr's Theory of The Hydrogen Atom - Physics IIDokument22 SeitenBohr's Theory of The Hydrogen Atom - Physics IISayyad aliNoch keine Bewertungen
- General Chemistry (CHEM F111) Lecture-11 13/04/2023Dokument15 SeitenGeneral Chemistry (CHEM F111) Lecture-11 13/04/2023Please Help BPHCNoch keine Bewertungen
- Hydrogen Atom and Non Euclidean GeometryDokument11 SeitenHydrogen Atom and Non Euclidean GeometryMichel MarcondesNoch keine Bewertungen
- Atomic and Molecular Spectroscopy Lecture 2Dokument29 SeitenAtomic and Molecular Spectroscopy Lecture 2Hammed LawalNoch keine Bewertungen
- 591 Notes v2 - 19Dokument230 Seiten591 Notes v2 - 19Michael Fralaide100% (1)
- CHEM2002 Unit Outline 2012Dokument8 SeitenCHEM2002 Unit Outline 2012Nishanthini SuppiahNoch keine Bewertungen
- Atomic Structure SRGP TestDokument18 SeitenAtomic Structure SRGP TestRinku Arun JainNoch keine Bewertungen
- IIT Question Papers 2007-2005Dokument92 SeitenIIT Question Papers 2007-2005CorneliaNoch keine Bewertungen
- Adam - Hall-Senior ThesisDokument103 SeitenAdam - Hall-Senior ThesisMo SaNoch keine Bewertungen
- 9Dokument4 Seiten9Bùi Hữu Đức0% (1)
- General Chemistry: CHEM F111Dokument29 SeitenGeneral Chemistry: CHEM F111Harsh TiwariNoch keine Bewertungen
- Atomic Structure and Atomic SpectraDokument37 SeitenAtomic Structure and Atomic SpectraAniSusiloNoch keine Bewertungen
- (2012) Relativistic Effects in Chemistry More Common Than You ThoughtDokument23 Seiten(2012) Relativistic Effects in Chemistry More Common Than You ThoughtBeatrizpioNoch keine Bewertungen
- Atomic Structure ModelsDokument49 SeitenAtomic Structure ModelsAshishNoch keine Bewertungen
- Lecture Notes: PhysicsDokument308 SeitenLecture Notes: Physicsdusk.foxiNoch keine Bewertungen
- Atomic Structure: Atomic Structure: Overview of Bohr's Atomic ModelDokument38 SeitenAtomic Structure: Atomic Structure: Overview of Bohr's Atomic Modelmanoj kumarNoch keine Bewertungen
- A. Z. Capri-Problems & Solutions in Nonrelativistic Quantum Mechanics-World Scientific (2002)Dokument507 SeitenA. Z. Capri-Problems & Solutions in Nonrelativistic Quantum Mechanics-World Scientific (2002)Miguel Angel Giménez67% (3)
- Hoja 2 PDFDokument3 SeitenHoja 2 PDFcris100% (1)
- Quantum Mechanics THIRD EDITION Eugene MerzbacherDokument670 SeitenQuantum Mechanics THIRD EDITION Eugene MerzbachersplouvrosNoch keine Bewertungen
- Problemi PropostiDokument4 SeitenProblemi PropostiDavide ZuccariniNoch keine Bewertungen
- Griffiths QMCH 4 P 19Dokument6 SeitenGriffiths QMCH 4 P 19Anushka Roy100% (1)
- Lecture Magnetism in Solids-Trinity College-JS3015Dokument115 SeitenLecture Magnetism in Solids-Trinity College-JS3015Anonymous 9rJe2lOskxNoch keine Bewertungen
- Lecture 09 PDFDokument25 SeitenLecture 09 PDFRachit ShahNoch keine Bewertungen
- Allen EXERCISE - (JEE Advance) Atomic Structure - CombinedDokument20 SeitenAllen EXERCISE - (JEE Advance) Atomic Structure - CombinedTejaswi JhaNoch keine Bewertungen
- Physical Chemistry Homework HelpDokument14 SeitenPhysical Chemistry Homework HelpEdu Assignment Help100% (1)
- Quantum Atomic and Molecular Physics ExplainedDokument67 SeitenQuantum Atomic and Molecular Physics ExplainedAndrew OrrNoch keine Bewertungen
- Practice Problems 3.0 - Atomic Structure JPP 1Dokument31 SeitenPractice Problems 3.0 - Atomic Structure JPP 1gangadaran buvanaNoch keine Bewertungen
- The Supersymmetric Dirac EquationDokument216 SeitenThe Supersymmetric Dirac EquationRama Krishna LankaNoch keine Bewertungen
- Atkins CH13 Atomic StructureDokument21 SeitenAtkins CH13 Atomic StructureAdarsh RameshNoch keine Bewertungen
- Ex 7Dokument5 SeitenEx 7Jean AraúzNoch keine Bewertungen