Beruflich Dokumente
Kultur Dokumente
A1113 PDF
Hochgeladen von
AjaykumarOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
A1113 PDF
Hochgeladen von
AjaykumarCopyright:
Verfügbare Formate
"OIL AND GAS PROCESSING PLANT DESIGN 2002
AND OPERATION TRAINING COURSE"
Page 32
DGEP/SCR/ED/ECP "GAS SWEETENING PROCESSES"
The process operates under the adsorption/regeneration cycle (generally 8 to 12 hours for both
sequences). Regeneration of the beds of sieves generally through the use of a side stream of
treated gas. Once the bed has been regerated (Its acid cpompounds having been removed), it is
ready for a new adsorption cycle.
When both H2S, CO2 and water vapor are present in the Feed Gas to the molecular sieves, the
water can promote the formation of COS (carbonyl sulfide) through inter reaction between CO2
and H2S (this sulfur compound can be a contaminant of the Treated Gas). High pressures, low
temperatures, high gas velocities tend to reduce the formation of COS. In this case, COS
depressant molecular sieve type must be considered .
Another problem relates to the disposal of the regeneration gas (mainly if used as fuel gas). The
cyclic nature of an adsorption operation could result in dumping peak CO2 and/or H2S loads in the
regeneration gas which would produce bumps as great as 30 to 40 times the initial acid gas
concentration.
Figure XI-B.14 shows a physical adsorption process to remove CO2 from a natural gas stream with
three molecular sieve vessels (one for the adsorption and the two other for the regeneration of the
sieves, one under heating the other under cooling).
2.5. Cryogenic Fractionation
2.5.1. Ryan Holmes
The cryogenic fractionation is a process for the removal of CO2 from Natural Gas. Should H2S be
present in the gas and should H2S removal be required, a dedicated process for H2S selective
removal would have to be provided upstream of the cryogenic fractionation. The cryogenic
fractionation does not remove H2S from Natural Gas.
This process originally developed by KOCH Process Systems Inc. was titled "RYAN/HOLMES
Process" after two employee-inventors and involves the use of a hydrocarbon additive usually
present in the Natural Gas to provide a particular benefit to the distillation (lowering the CO2
freezing point). The "RYAN/HOLMES Process" uses Natural Gas Liquid (NGL) which is extracted
from the feed stream itself.
Figure XI-B.14 shows the principle of the "RYAN/HOLMES Process".
The following two principal distillative separations are involved:
. Separation of methane from CO2. This separation uses the NGL stream to avoid CO2
freezing and takes place in the "RYAN/HOLMES demethanizer".
. Separation of CO2 from ethane plus (C2+). This separation uses the NGL stream to break
the CO2/ethane azeotrope.
Excerpt from PRODEM
"OIL AND GAS PROCESSING PLANT DESIGN 2002
AND OPERATION TRAINING COURSE"
Page 33
DGEP/SCR/ED/ECP "GAS SWEETENING PROCESSES"
Since this fractionation require low temperatures, its best utilization is the LNG manufacture.
However, none of the existing LNG trains in the word to date uses this process. This process is no
longer developped by KOCH and thus falls in the public domain.
Derived cryogenic processes working at temperatures above the CO2 solidification point have
been implemented for the bulk removal of CO2 from natural gas.
2.5.2. SPREX
The SPREX is a process under development by TFE and IFP. It is dedicated to very sour gas with
typically 40 % H2S content for bulk removal of this acid gas and re-injection in wells.
It mainly consists in a distillation column the reflux of which is ensured at typically –30°C by a
refrigeration unit and supplied with a reboiler. The H2S / CO2 mixture is produced at the bottom of
the column in a liquid phase. It can be pumped to high pressure for well injection.
The top gas leaves the column with a remaining typical content of 10 % H2S and can be routed to
a classical MDEA process.
A pilot unit is to be erected in the Lacq plant for test and operation demonstration.
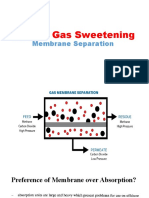
2.6. Permeation (Membrane)
Polymeric membrane (which contains no hole or pore) is not a new technology for gas separation.
This separation is based on the principle that certain gas compounds dissolve and diffuse through
the polymeric material at a faster rate than others. Carbon dioxide, hydrogen, helium, hydrogen
sulfide and water vapor are highly permeable (« fast gases »). Conversely, nitrogen, methane and
heavier paraffin compounds are less permeable (« slow gases »). The best application for
membrane separation is to separate gases in the « fast » category (Permeate) from gases in the
« slow » category (Residue Gas). For instance, CO2 will pass through a polymer membrane 15 to
40 times faster than methane.
Residue Gas from the membranes is at a pressure close to that of the Feed Gas to the
membranes whilst the Permeate is at low pressure (between 1 and 4 atmosphere absolute).
Membrane is then an attractive process to remove CO2 (bulk removal) and traces of H2S
from natural gas. H2S removal when present in high concentrations (in terms of mole %) is not
applicable due to the detrimental effect of this compound on the membrane.
Theoretically the Permeation process has no rotating equipment and no energy requirement.
However this technology cannot allow to satisfy Sales Gas specifications or LNG specifications
(H2S lower than 4 ppm V and CO2 less than 90 ppm V). Its application is for the bulk removal
of CO2 from nearly H2S free natural gases.
The membrane also removes water from the feed gas.
Excerpt from PRODEM
"OIL AND GAS PROCESSING PLANT DESIGN 2002
AND OPERATION TRAINING COURSE"
Page 34
DGEP/SCR/ED/ECP "GAS SWEETENING PROCESSES"
The permeation phenomenon occurs in four (4) steps :
. Adsorption of the CO2 by the active surface of the membrane (at the raw gas
pressure)
. Dissolution of the CO2 in the membrane .
. Diffusion of the CO2 through the membrane .
. Desorption of the CO2 from the membrane (at low pressure) .
The adsorption of the CO2 is better at high pressure (high CO2 partial pressures). This process is
then not well suited to low pressure operations.
The polymeric material to be selected for membrane construction must be permeable to CO2 but
also selective (to avoid permeation of hydrocarbon gas components).
There are two (2) types of membranes :
. Spiral Wound membrane .
. Hollow fiber membrane .
The main manufacturers of membranes are :
. CYNARA (formerly DOW group, thereafter belonging to HELER group and now in the
NATCO group) using Hollow Fiber membranes. These membranes are based on the
use of a cellulose polymer (cellulose triacetate). This Licenser provided its first plant
in 1983 (Sacroc plant in Texas).
A large off shore plant (PAILIN in THAILAND) operated by UNOCAL equipped with
CYNARA membranes is under construction.
CYNARA membrane can be also used to remove CO2 from light liquid. One plant
(Marathon’s Burns Point gas plant in St-Mary Parrish in southern Louisiana) uses
CYNARA membrane for NGL (natural Gas Liquid stream) sweetening (3000 BPD of
NGL with 11.7 mole % CO2 inlet and 5 mole % max. outlet).
Due to the fact that their current membranes cannot withstand high pressures (less
than 100 b), CYNARA is currently testing more robust types of membranes (polyimide
and other kind of cellulose acetate).
The membrane is fitted in a vertical tube. Each tube is vertically installed. This is an
advantage for the natural drainage of the condensed liquid which can form due to the
displacement of the treated gas hydrocarbon dew curve (reduction of the CO2
content). CYNARA can provide tube with a diameter of 16 inch for a length of 2 m.
. KVAERNER (formerly GRACE).
GRACE originally developed membranes on the Spiral Wound technology (cellulose
acetate) from the beginning 1984 to July 1993 date at which they change to polyimide
The membrane unit can include several modules (a module is a shell which
accommodates the membranes). There are 6 or 7 membranes per module. Each
module in horizontally mounted. Hollow Fiber or Spiral Wound membranes can be
fitted in the same module.
From 1990 up to 1993, GRACE has already provided more than 80 acid gas removal
(mainly CO2) units (capacity ranging from 0.5 MMSCFD to 20 MMSCFD) based on
their old spiral wound technology. After 1993 up to 1996, with the change to hollow
Excerpt from PRODEM
Das könnte Ihnen auch gefallen
- Gas Sweetening ProcessesDokument9 SeitenGas Sweetening ProcessesAjaykumarNoch keine Bewertungen
- A1114 PDFDokument4 SeitenA1114 PDFAjaykumarNoch keine Bewertungen
- Natural Gas Engineering: Adsorption and Membranes for Acid Gas RemovalDokument17 SeitenNatural Gas Engineering: Adsorption and Membranes for Acid Gas RemovalSahil PatilNoch keine Bewertungen
- Experiences in The OperationDokument7 SeitenExperiences in The OperationUsama Bin SabirNoch keine Bewertungen
- Paper 1Dokument12 SeitenPaper 1Payam ParvasiNoch keine Bewertungen
- Article 123Dokument14 SeitenArticle 123Jumana SharanikNoch keine Bewertungen
- Desiccants PDFDokument9 SeitenDesiccants PDFjesus_manrique2753Noch keine Bewertungen
- Gas Sweetening ProcessesDokument4 SeitenGas Sweetening ProcessesAjaykumarNoch keine Bewertungen
- Adsorption Process For Natural GAS TreatmentDokument24 SeitenAdsorption Process For Natural GAS TreatmentAhmed ElShora100% (3)
- Adsorption TechnologiesDokument21 SeitenAdsorption TechnologiesDhruv BajpaiNoch keine Bewertungen
- HYSYS Simulation For Natural Gas Dehydration and SweeteningDokument8 SeitenHYSYS Simulation For Natural Gas Dehydration and SweeteningHaziq MirzaNoch keine Bewertungen
- CO2 Capture at Ambient Temperature in A Fixed Bed With CaO Based Sorbents 2015 Applied EnergyDokument7 SeitenCO2 Capture at Ambient Temperature in A Fixed Bed With CaO Based Sorbents 2015 Applied EnergyserchNoch keine Bewertungen
- Aspen-HYSYS simulation of natural gas processingDokument4 SeitenAspen-HYSYS simulation of natural gas processingcandraNoch keine Bewertungen
- Aspen-HYSYS Simulation of Natural Gas Processing PlantDokument4 SeitenAspen-HYSYS Simulation of Natural Gas Processing Plantsorincarmen88Noch keine Bewertungen
- Absorber TowerDokument14 SeitenAbsorber TowerAhmad Fariz Maulana100% (1)
- 児玉先生熱交換論文Dokument9 Seiten児玉先生熱交換論文Long TranNoch keine Bewertungen
- Accepted ManuscriptDokument29 SeitenAccepted ManuscriptAlek KrótkiNoch keine Bewertungen
- MOF-based TSA cycle for CO2 capture from wet flue gasDokument15 SeitenMOF-based TSA cycle for CO2 capture from wet flue gasAdam MNoch keine Bewertungen
- Application of A Chilled Ammonia-Based Process For CO Capture To Cement PlantsDokument9 SeitenApplication of A Chilled Ammonia-Based Process For CO Capture To Cement PlantsAkash NamdeoNoch keine Bewertungen
- Technologies To Reduce Air PollutionDokument18 SeitenTechnologies To Reduce Air PollutionSambiri PisiraiNoch keine Bewertungen
- Pressurized Glycol Dehy SystemsDokument11 SeitenPressurized Glycol Dehy SystemsJagan BoseNoch keine Bewertungen
- Traitment de GazDokument6 SeitenTraitment de GazDjamel Eddine MekkiNoch keine Bewertungen
- Natural Gas Sweetening 2Dokument16 SeitenNatural Gas Sweetening 2MALIK ZARYABBABARNoch keine Bewertungen
- Integrated Catalytic Membrane Reactor For Hydrogen Production Using Hydrocarbon-Based FuelsDokument4 SeitenIntegrated Catalytic Membrane Reactor For Hydrogen Production Using Hydrocarbon-Based FuelsMazen OthmanNoch keine Bewertungen
- Carbon Capture and StorageDokument24 SeitenCarbon Capture and StorageShashwat OmarNoch keine Bewertungen
- Hybrid System-An Emerging Solution To Sour Gas TreatmentDokument7 SeitenHybrid System-An Emerging Solution To Sour Gas TreatmentفتحيNoch keine Bewertungen
- Gas DehydrationDokument14 SeitenGas DehydrationKwesi EdwardsNoch keine Bewertungen
- Sedl-Kov Membranes For BiomethaneDokument8 SeitenSedl-Kov Membranes For BiomethaneJuan Sebastián Espinosa EscobarNoch keine Bewertungen
- Gas Pre TreatmentDokument12 SeitenGas Pre TreatmentAnonymous bHh1L1100% (4)
- Natural Gas ProcessingDokument24 SeitenNatural Gas ProcessingMuhammad Shariq KhanNoch keine Bewertungen
- Sulfur Magazine Ideas For Better Clean Up Jan 09Dokument0 SeitenSulfur Magazine Ideas For Better Clean Up Jan 09Bharat VaajNoch keine Bewertungen
- Improving The Efficiency of A Chilled Ammonia CO2Dokument7 SeitenImproving The Efficiency of A Chilled Ammonia CO2ناصر احمدNoch keine Bewertungen
- Powerplant G3Dokument45 SeitenPowerplant G3Animesh Kr. AmarNoch keine Bewertungen
- CO2 Separation TechnologiesDokument14 SeitenCO2 Separation TechnologiesbltzkrigNoch keine Bewertungen
- CO2 Separation by Membrane Technologies: Applications and PotentialitiesDokument6 SeitenCO2 Separation by Membrane Technologies: Applications and Potentialitiesjeffbush23Noch keine Bewertungen
- Natural-Gas Processing Is A Complex Industrial Process Designed To Clean Raw Natural Gas byDokument4 SeitenNatural-Gas Processing Is A Complex Industrial Process Designed To Clean Raw Natural Gas byLavenia Alou MagnoNoch keine Bewertungen
- Uraian Proses AlcoholDokument7 SeitenUraian Proses Alcoholryan_mardianoNoch keine Bewertungen
- Carbon Capture and StorageDokument32 SeitenCarbon Capture and StorageAyush BaliNoch keine Bewertungen
- Gas Sweetening Processes: Aqueous Solution Wt. % Mole % Mdea 50 13.13 H2O 50 86.87 Total 100 100.00Dokument3 SeitenGas Sweetening Processes: Aqueous Solution Wt. % Mole % Mdea 50 13.13 H2O 50 86.87 Total 100 100.00AjaykumarNoch keine Bewertungen
- Scientific & Technical ReportDokument15 SeitenScientific & Technical ReportMariam AsgharNoch keine Bewertungen
- Preparation of Sorbents. The PotassiumDokument5 SeitenPreparation of Sorbents. The PotassiumFarah TalibNoch keine Bewertungen
- SulphurRecovery PDFDokument22 SeitenSulphurRecovery PDFfatamorgganaNoch keine Bewertungen
- Benefits of deliquescing desiccants for gas dehydrationDokument8 SeitenBenefits of deliquescing desiccants for gas dehydrationcclaremontNoch keine Bewertungen
- Economic N2 Removal Hydrocarbon EngineeringDokument8 SeitenEconomic N2 Removal Hydrocarbon EngineeringSergio JavierNoch keine Bewertungen
- Combined-Cycle HRSG Shutdown, Layup, and Startup Chemistry Control - POWERDokument15 SeitenCombined-Cycle HRSG Shutdown, Layup, and Startup Chemistry Control - POWERShameer MajeedNoch keine Bewertungen
- Absorption Cap 1Dokument2 SeitenAbsorption Cap 1alfonso.gonzalez68Noch keine Bewertungen
- AZEP - Development of An Integrated Air Separation Membrane - Gas TurbineDokument6 SeitenAZEP - Development of An Integrated Air Separation Membrane - Gas TurbineeddyNoch keine Bewertungen
- Environmental Protection in The Field of Thermal Power PlantDokument21 SeitenEnvironmental Protection in The Field of Thermal Power PlantGoverdhan ShresthaNoch keine Bewertungen
- Interactive Gas Processing Portfolio FinalDokument80 SeitenInteractive Gas Processing Portfolio FinalDeepakNoch keine Bewertungen
- Natural Gas: Operations and Transport: A Handbook for Students of the Natural Gas IndustryVon EverandNatural Gas: Operations and Transport: A Handbook for Students of the Natural Gas IndustryNoch keine Bewertungen
- Carbon Capture Technologies for Gas-Turbine-Based Power PlantsVon EverandCarbon Capture Technologies for Gas-Turbine-Based Power PlantsNoch keine Bewertungen
- Carbon Capture and Storage: The Legal Landscape of Climate Change Mitigation TechnologyVon EverandCarbon Capture and Storage: The Legal Landscape of Climate Change Mitigation TechnologyNoch keine Bewertungen
- Corrosion and Fouling Control in Desalination IndustryVon EverandCorrosion and Fouling Control in Desalination IndustryNoch keine Bewertungen
- Emulsions and Oil Treating Equipment: Selection, Sizing and TroubleshootingVon EverandEmulsions and Oil Treating Equipment: Selection, Sizing and TroubleshootingBewertung: 5 von 5 Sternen5/5 (3)
- Clean Ironmaking and Steelmaking Processes: Efficient Technologies for Greenhouse Emissions AbatementVon EverandClean Ironmaking and Steelmaking Processes: Efficient Technologies for Greenhouse Emissions AbatementNoch keine Bewertungen
- Encyclopaedia Britannica, 11th Edition, Volume 8, Slice 3 "Destructors" to "Diameter"Von EverandEncyclopaedia Britannica, 11th Edition, Volume 8, Slice 3 "Destructors" to "Diameter"Noch keine Bewertungen
- Integrated Sand Management For Effective Hydrocarbon Flow AssuranceVon EverandIntegrated Sand Management For Effective Hydrocarbon Flow AssuranceNoch keine Bewertungen
- Gas Sweetening Processes: 3. BibliographyDokument1 SeiteGas Sweetening Processes: 3. BibliographyAjaykumarNoch keine Bewertungen
- Catalysts: Olefins From Biomass Intermediates: A ReviewDokument19 SeitenCatalysts: Olefins From Biomass Intermediates: A ReviewfadyahNoch keine Bewertungen
- Polypropylene: PERP 2011-5Dokument6 SeitenPolypropylene: PERP 2011-5AjaykumarNoch keine Bewertungen
- Gas Sweetening Process Selection GuideDokument1 SeiteGas Sweetening Process Selection GuideAjaykumarNoch keine Bewertungen
- Total/Uop Olefin Cracking Process: EthyleneDokument1 SeiteTotal/Uop Olefin Cracking Process: EthyleneAjaykumarNoch keine Bewertungen
- Gas Sweetening Processes ExplainedDokument3 SeitenGas Sweetening Processes ExplainedAjaykumarNoch keine Bewertungen
- Overview of Worldwide Technologies of Pyrolysis and Perspective of DevelopmentDokument8 SeitenOverview of Worldwide Technologies of Pyrolysis and Perspective of DevelopmentIman GhaleeNoch keine Bewertungen
- EP0833155A1Dokument18 SeitenEP0833155A1AjaykumarNoch keine Bewertungen
- Gas Sweetening ProcessesDokument4 SeitenGas Sweetening ProcessesAjaykumarNoch keine Bewertungen
- Polypropylene: PERP 2011-5Dokument6 SeitenPolypropylene: PERP 2011-5AjaykumarNoch keine Bewertungen
- Gas Sweetening ProcessesDokument5 SeitenGas Sweetening ProcessesAjaykumarNoch keine Bewertungen
- "Oil and Gas Processing Plant Design and Operation Training Course" " " 2002Dokument10 Seiten"Oil and Gas Processing Plant Design and Operation Training Course" " " 2002AjaykumarNoch keine Bewertungen
- Gas Processing I - Chapter 3 Rev 1Dokument26 SeitenGas Processing I - Chapter 3 Rev 1ABULARA2K6Noch keine Bewertungen
- PPIV PLANT OverviewDokument52 SeitenPPIV PLANT OverviewShreya Shwetima100% (2)
- PONA Analysis On AB-PONA ColumnDokument7 SeitenPONA Analysis On AB-PONA ColumnAjaykumarNoch keine Bewertungen
- Cooling water, steam, air and power requirements for 275 KTA PP plantDokument5 SeitenCooling water, steam, air and power requirements for 275 KTA PP plantAjaykumarNoch keine Bewertungen
- Annajaf City Naphtha As A Commercial Alternative Fuel For S.I. Engines in IraqDokument10 SeitenAnnajaf City Naphtha As A Commercial Alternative Fuel For S.I. Engines in IraqAjaykumarNoch keine Bewertungen
- Student Lecture 11 Field Processing and Treatment of Natural GasDokument38 SeitenStudent Lecture 11 Field Processing and Treatment of Natural GasAjaykumarNoch keine Bewertungen
- Polypropylene PDFDokument36 SeitenPolypropylene PDFabdul qahar100% (1)
- Melt Flow Index ControlDokument8 SeitenMelt Flow Index ControlAjaykumarNoch keine Bewertungen
- Olefine TechnologyDokument1 SeiteOlefine TechnologyAjaykumarNoch keine Bewertungen
- The Star Process by Uhde: Industrial SolutionsDokument24 SeitenThe Star Process by Uhde: Industrial SolutionsAjaykumarNoch keine Bewertungen
- Found Support - Reading - Lesson 8Dokument8 SeitenFound Support - Reading - Lesson 8Hiếu HiếuNoch keine Bewertungen
- Festo OD TubingDokument40 SeitenFesto OD TubingyogitatanavadeNoch keine Bewertungen
- Chemkin PDFDokument44 SeitenChemkin PDFmohamedIGCMONoch keine Bewertungen
- Fire Retardant Spray ComparisonDokument11 SeitenFire Retardant Spray Comparisonsteve4goshel100% (1)
- Ra 9003Dokument101 SeitenRa 9003Charles Rommel TadoNoch keine Bewertungen
- SKYTEC Datasheet TEAL 0723Dokument2 SeitenSKYTEC Datasheet TEAL 0723mailtoshiyasNoch keine Bewertungen
- Aroma Chemistry Smell of BooksDokument1 SeiteAroma Chemistry Smell of BooksEstefanía Gómez RodríguezNoch keine Bewertungen
- CH 5 Coordination CompoundsDokument53 SeitenCH 5 Coordination Compoundsgerawop972Noch keine Bewertungen
- Readymix ProfileDokument33 SeitenReadymix ProfileAyman HammodehNoch keine Bewertungen
- Electric Water Heaters GuideDokument36 SeitenElectric Water Heaters GuidearshadNoch keine Bewertungen
- Painting Inspection Procedure PDFDokument1 SeitePainting Inspection Procedure PDFPhạm Văn Đảng67% (3)
- Bro-0008.7 Hvofsolutions enDokument16 SeitenBro-0008.7 Hvofsolutions enIzziNoch keine Bewertungen
- The First Law of ThermodynamicsDokument10 SeitenThe First Law of ThermodynamicsArpit PatelNoch keine Bewertungen
- Advanced Silicide Based Materials - MoSi2Dokument13 SeitenAdvanced Silicide Based Materials - MoSi2chandravadiyaketanNoch keine Bewertungen
- Handling of MaterialsDokument34 SeitenHandling of MaterialsJerome GarganeraNoch keine Bewertungen
- PREFORMULATION STUDIEsDokument8 SeitenPREFORMULATION STUDIEsTanishaNoch keine Bewertungen
- Annex II - AMC 20-29Dokument36 SeitenAnnex II - AMC 20-29Victor Manuel Peña EsparteroNoch keine Bewertungen
- 3LW - Types and Properties of Aviation OilsDokument10 Seiten3LW - Types and Properties of Aviation OilsZouhair ElmNoch keine Bewertungen
- Historical Development of The Periodic TableDokument12 SeitenHistorical Development of The Periodic TableWan HasliraNoch keine Bewertungen
- Praktikum Metal 1Dokument27 SeitenPraktikum Metal 1Muhammad Aldi Luthfi FauzanNoch keine Bewertungen
- Refinery Presentation On 07th July 07Dokument83 SeitenRefinery Presentation On 07th July 07meenuNoch keine Bewertungen
- Wilma Ty Nueva SpecificationsDokument10 SeitenWilma Ty Nueva SpecificationsJubs DejitoNoch keine Bewertungen
- A-Ele-Lst-000-47960-B - Cable Shedule For Pipeline Cathodic Protection SystemDokument44 SeitenA-Ele-Lst-000-47960-B - Cable Shedule For Pipeline Cathodic Protection SystemBadiNoch keine Bewertungen
- HVOF Processed CoCrFeMnNiDokument11 SeitenHVOF Processed CoCrFeMnNiRafael RiveraNoch keine Bewertungen
- Quarter 2: Week 11 Lo 3. Handle Materials and Equipment TLE-AFAC10CW-11a-e-3Dokument16 SeitenQuarter 2: Week 11 Lo 3. Handle Materials and Equipment TLE-AFAC10CW-11a-e-3Romeo Jr Vicente Ramirez100% (5)
- Subgrade Rutting Flexible PavementDokument5 SeitenSubgrade Rutting Flexible PavementPalak ShivhareNoch keine Bewertungen
- Building The Formula For Calculating The Vapor of The LPG Liquid Generating in The Explosion Risk of LPG TankDokument3 SeitenBuilding The Formula For Calculating The Vapor of The LPG Liquid Generating in The Explosion Risk of LPG TankLy Ngoc MinhNoch keine Bewertungen
- Eq Tip No. Short Column Effect: Prepared by ContactDokument24 SeitenEq Tip No. Short Column Effect: Prepared by ContactNasshikin RamliNoch keine Bewertungen
- Bulletin ASTM C 920Dokument1 SeiteBulletin ASTM C 920carlosNoch keine Bewertungen
- BOQ - Hearts & Arrows Office 04sep2023Dokument15 SeitenBOQ - Hearts & Arrows Office 04sep2023ChristianNoch keine Bewertungen