Beruflich Dokumente
Kultur Dokumente
4 Acids, Bases and Salts
Hochgeladen von
Ahnt htoo aungOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
4 Acids, Bases and Salts
Hochgeladen von
Ahnt htoo aungCopyright:
Verfügbare Formate
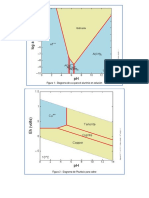
4 Acids, bases and salts
Cu Cu O 12
C Ar = 12 0 1 2 3 4 5 6
+ O O
H+ acids
H+ OH –
Cu Cu O Cu O Mr = 80 Masses of compounds methyl orange
8 9 10 11 12 13 14
ACID + BASE
FeCl3 + 3NaOH Fe(OH)3 + 3NaCl OH – bases
SALT + WATER
4.1 Stoichiometry 8.1 The characteristic properties
of acids and bases
Equations
Uses
Neutralisation reactions
nitrogen NH4NO3
HCl
+ NaOH NaCl
+ H2O
phosphorus PO43–
potassium KNO3
Salts and their uses
ACID + BASE SALT + WATER Ca(OH)2 + 2NH4Cl 2NH3 + CaCl2 + H2O
8.3 Preparation of salts 11.3 Nitrogen and fertilisers
Reactions of carbonates and acids
CuCO 3 CO 2 CaO
metal elements
non-metal elements
CaCO3
CaCO3
Thermal decomposition
Na2O MgO ZnO CO2 NO2 SO2
Ca(OH)2
heat CuO
Indicators
8.2 Types of oxides 13.1 Carbonates
AgNO3
Cu2+ Na+ K+ Li+ Cl– Br– I–
Testing for ions and gases Testing for carbonates
O2 Cl2
CO2 NH3 H2
8.4 Identification of ions and gases
Copyright © UCLES 2017
Das könnte Ihnen auch gefallen
- Naming and Writing Chemical FormulaDokument8 SeitenNaming and Writing Chemical FormulaMarie Angeline BautistaNoch keine Bewertungen
- IonicBonding WritingFormulas WKST KEYDokument2 SeitenIonicBonding WritingFormulas WKST KEYMaria Isabel DicoNoch keine Bewertungen
- Basic Diagrams Intro 2020Dokument19 SeitenBasic Diagrams Intro 2020Büşra DOĞRUNoch keine Bewertungen
- 0620 - 04 Acids, Bases and SaltsDokument214 Seiten0620 - 04 Acids, Bases and SaltsShivamNoch keine Bewertungen
- Acids, Bases and Salts PDFDokument1 SeiteAcids, Bases and Salts PDFZeyad OsamaNoch keine Bewertungen
- Hydrolysis ReadDokument4 SeitenHydrolysis ReadJohn LionelNoch keine Bewertungen
- PT With POLY IONS Revise 11-17-08Dokument1 SeitePT With POLY IONS Revise 11-17-08FFFNoch keine Bewertungen
- Important TablesDokument6 SeitenImportant TablesMaheswariNoch keine Bewertungen
- Key Chem TheoryDokument8 SeitenKey Chem Theoryalex.holdcroft23Noch keine Bewertungen
- Acids and Bases YMS X ALJDokument9 SeitenAcids and Bases YMS X ALJFaqihah Syahindah Mohammed FiroozNoch keine Bewertungen
- REACTIVO 2 TermodinamicaDokument5 SeitenREACTIVO 2 TermodinamicaCalvin JacobNoch keine Bewertungen
- Development of Baking Powder: Landmark Lesson PlanDokument17 SeitenDevelopment of Baking Powder: Landmark Lesson PlanAtom NerdNoch keine Bewertungen
- Periodic Table of Scaling Compounds: Caso SioDokument2 SeitenPeriodic Table of Scaling Compounds: Caso SioManiKantan GangatharanNoch keine Bewertungen
- Kisi-Kisi Mid-Test BiologyDokument3 SeitenKisi-Kisi Mid-Test BiologyIda FaridaNoch keine Bewertungen
- Lecture 15Dokument24 SeitenLecture 15Muhammad AbdullahNoch keine Bewertungen
- Solubility ProductsDokument2 SeitenSolubility Productsgracemizzi6Noch keine Bewertungen
- Chemistry 1302: CHAPTER 3.3 - Acid Base ChemistryDokument15 SeitenChemistry 1302: CHAPTER 3.3 - Acid Base Chemistryzak mahmoudNoch keine Bewertungen
- 1st Sec Catalyst 3 Unit 2Dokument1 Seite1st Sec Catalyst 3 Unit 2yassinyaser95Noch keine Bewertungen
- Today's Plan: Salts, Acids and BasesDokument5 SeitenToday's Plan: Salts, Acids and Basesvinnie0905Noch keine Bewertungen
- Preparation Properties Fi PDFDokument43 SeitenPreparation Properties Fi PDFPlaying PUBGNoch keine Bewertungen
- Unit 08b: Advanced Hydrogeology: Groundwater ChemistryDokument22 SeitenUnit 08b: Advanced Hydrogeology: Groundwater ChemistryWellfroNoch keine Bewertungen
- 22 - Carboxylic Acids & Esters CORNELLDokument12 Seiten22 - Carboxylic Acids & Esters CORNELLGeorge SolomouNoch keine Bewertungen
- A2-Organic Reactions Spider Diagram HANDOUT (Colour)Dokument1 SeiteA2-Organic Reactions Spider Diagram HANDOUT (Colour)udaymohur100% (1)
- Carboxylic & DerivtDokument7 SeitenCarboxylic & DerivtNanda NaimahNoch keine Bewertungen
- Table of Polyatomic IonsDokument3 SeitenTable of Polyatomic IonsBradyNoch keine Bewertungen
- Recovery Boiler Chemical PrinciplesDokument18 SeitenRecovery Boiler Chemical PrinciplesAA MAXNoch keine Bewertungen
- Chemistry, C8A - Aanotes (S)Dokument26 SeitenChemistry, C8A - Aanotes (S)Farah Aisyah AhmadNoch keine Bewertungen
- A L C P I: Lphabetical Ist of Ommon Olyatomic ONS N F C +1Dokument2 SeitenA L C P I: Lphabetical Ist of Ommon Olyatomic ONS N F C +1RedHoeBeerNoch keine Bewertungen
- Sulfuric Acid Production Sulfuric Acid: By: Carl Cesar H. BibatDokument7 SeitenSulfuric Acid Production Sulfuric Acid: By: Carl Cesar H. BibatSam Denielle TugaoenNoch keine Bewertungen
- OXIDES (Metals & Non-Metals)Dokument4 SeitenOXIDES (Metals & Non-Metals)gauri guptaNoch keine Bewertungen
- Electrical Double Layer Cu20Dokument14 SeitenElectrical Double Layer Cu20Anonymous PT1b9IWNoch keine Bewertungen
- PCM Chapter 01 Part BDokument7 SeitenPCM Chapter 01 Part BAlif AzmirNoch keine Bewertungen
- Fongrsy - Acids Bases and AlkalisDokument2 SeitenFongrsy - Acids Bases and AlkalisDinangaNoch keine Bewertungen
- SALTSDokument7 SeitenSALTSNelson UgwuNoch keine Bewertungen
- Chem f4 (SALTS)Dokument26 SeitenChem f4 (SALTS)nur asyiqinNoch keine Bewertungen
- Chemical Formulas List For Class 10 - PDF - Hydroxide - OxideDokument1 SeiteChemical Formulas List For Class 10 - PDF - Hydroxide - OxidedohareykalpanaNoch keine Bewertungen
- Direct Extraction of Copper From Copper Sulfide Minerals Using Deep Eutectic SolventsDokument3 SeitenDirect Extraction of Copper From Copper Sulfide Minerals Using Deep Eutectic SolventsFloren Ardila AlvaradoNoch keine Bewertungen
- !chemistry Review ANSDokument3 Seiten!chemistry Review ANSAngel LiNoch keine Bewertungen
- GCSE KO Formuale and EquationsDokument1 SeiteGCSE KO Formuale and EquationsPaulina MugutiNoch keine Bewertungen
- Gráficas GeochemDokument5 SeitenGráficas Geochemvandrake10Noch keine Bewertungen
- Support Chimie Descriptive LST-CA 2023Dokument52 SeitenSupport Chimie Descriptive LST-CA 2023salmayassine203Noch keine Bewertungen
- Road Maps Organic Chemistry Set 3 Eklavya @JEEAdvanced - 2024Dokument6 SeitenRoad Maps Organic Chemistry Set 3 Eklavya @JEEAdvanced - 2024puneethrgcNoch keine Bewertungen
- Compound RulesDokument5 SeitenCompound RulesNa YoungNoch keine Bewertungen
- Chemistry F4 SaltsDokument13 SeitenChemistry F4 Saltscivichitam18Noch keine Bewertungen
- Lecture 7 - Stability DiagramsDokument7 SeitenLecture 7 - Stability DiagramsAththur MaulanaNoch keine Bewertungen
- Lesson Element Making Salts: Instructions and Answers For TeachersDokument17 SeitenLesson Element Making Salts: Instructions and Answers For TeachersGracey- Ann JohnsonNoch keine Bewertungen
- CO2 Absorbance and WaterDokument9 SeitenCO2 Absorbance and WaterNaveed MubarikNoch keine Bewertungen
- Che 176 Carboxylic Acids-2Dokument67 SeitenChe 176 Carboxylic Acids-2BalogunNoch keine Bewertungen
- Chemistry SummaryDokument22 SeitenChemistry SummaryEmma Isabella GraceNoch keine Bewertungen
- Acids, Bases, Salts-HWNDokument4 SeitenAcids, Bases, Salts-HWNShelin GaziNoch keine Bewertungen
- Monatomic and Polyatomic IonsDokument2 SeitenMonatomic and Polyatomic Ionsaku 223Noch keine Bewertungen
- Acids Bases and SaltsDokument45 SeitenAcids Bases and SaltsTejas PagarNoch keine Bewertungen
- Sarah Balancing Chemical Equations Practice Paper 2023Dokument3 SeitenSarah Balancing Chemical Equations Practice Paper 2023sarah mukaddamNoch keine Bewertungen
- A B C D Answer: B, A, D, C: O O BR 1 Eq Br2 Febr3Dokument3 SeitenA B C D Answer: B, A, D, C: O O BR 1 Eq Br2 Febr3Quốc NguyễnNoch keine Bewertungen
- Analysis of Cations: - Ions, Which Form Compounds, Having Similar Properties Are Placed in A Single GroupDokument3 SeitenAnalysis of Cations: - Ions, Which Form Compounds, Having Similar Properties Are Placed in A Single GroupJan MezoNoch keine Bewertungen
- Complete Periodic Table 6Dokument9 SeitenComplete Periodic Table 6Fenil KoratNoch keine Bewertungen
- Limescale ChemistryDokument1 SeiteLimescale ChemistrygurlavatarNoch keine Bewertungen
- Inorganic Qualitative Analysis Acidic RadicalDokument24 SeitenInorganic Qualitative Analysis Acidic RadicalShivani ShreshthaNoch keine Bewertungen
- Atmospheric Chemical Compounds: Sources, Occurrence and BioassayVon EverandAtmospheric Chemical Compounds: Sources, Occurrence and BioassayNoch keine Bewertungen
- Polar Plunge, Wisconsin: By: Ahnt Htoo AungDokument6 SeitenPolar Plunge, Wisconsin: By: Ahnt Htoo AungAhnt htoo aungNoch keine Bewertungen
- S3 THM Chemistry CA2 20 - 21Dokument6 SeitenS3 THM Chemistry CA2 20 - 21Ahnt htoo aungNoch keine Bewertungen
- CitationsDokument2 SeitenCitationsAhnt htoo aungNoch keine Bewertungen
- How Does The Loss of Biodiversity Affect The Ecosystem?Dokument3 SeitenHow Does The Loss of Biodiversity Affect The Ecosystem?Ahnt htoo aungNoch keine Bewertungen
- PT-1 (CW-13) Question PaperDokument3 SeitenPT-1 (CW-13) Question PaperAhnt htoo aungNoch keine Bewertungen
- What Is The Ocean 2. What Caused The Problem 3. The Problem 4. How To Solve The ProblemDokument1 SeiteWhat Is The Ocean 2. What Caused The Problem 3. The Problem 4. How To Solve The ProblemAhnt htoo aungNoch keine Bewertungen
- How Are Humans Related To Ocean Pollution?: The IntroductionDokument9 SeitenHow Are Humans Related To Ocean Pollution?: The IntroductionAhnt htoo aungNoch keine Bewertungen
- Square-Wave VoltammetryDokument15 SeitenSquare-Wave VoltammetryNatasa VukicevicNoch keine Bewertungen
- CalorimetryDokument54 SeitenCalorimetryTrixsha TapsirulNoch keine Bewertungen
- CHE506 - Lab Report On Plug Flow ReactorDokument25 SeitenCHE506 - Lab Report On Plug Flow Reactorfiorella50% (2)
- Surface TensionDokument50 SeitenSurface TensionbagheldhirendraNoch keine Bewertungen
- OxidesDokument27 SeitenOxidesJuan KorNoch keine Bewertungen
- Hydrolysis-Condensation Processes of The Tetra-Alkoxysilanes TPOS, TEOS and TMOS in Some Alcoholic SolventsDokument13 SeitenHydrolysis-Condensation Processes of The Tetra-Alkoxysilanes TPOS, TEOS and TMOS in Some Alcoholic SolventssorescuNoch keine Bewertungen
- Pre FormulationDokument53 SeitenPre FormulationRubaba Rahman Abanti0% (1)
- Ejemplo DTML Crossflow PDFDokument2 SeitenEjemplo DTML Crossflow PDFDaniel González Juárez100% (1)
- Annual Exam - Class 11 - Chemistry Question PaperDokument4 SeitenAnnual Exam - Class 11 - Chemistry Question PaperADITIYANoch keine Bewertungen
- Handbook of Polymer BlendsDokument612 SeitenHandbook of Polymer BlendsKaihuanZhangNoch keine Bewertungen
- Fluid Package EOSDokument12 SeitenFluid Package EOSdani2611Noch keine Bewertungen
- 2 ND Michlaslab 402Dokument4 Seiten2 ND Michlaslab 402michialdasNoch keine Bewertungen
- Properties: 118, Optical of CopperDokument10 SeitenProperties: 118, Optical of CopperArun ArumugamNoch keine Bewertungen
- As Level Chemistry: Paddington Academy 1Dokument10 SeitenAs Level Chemistry: Paddington Academy 1Shahnaz AhmedNoch keine Bewertungen
- Dehydrobromination of Meso-Stilbene DibromideDokument8 SeitenDehydrobromination of Meso-Stilbene DibromideMo MlNoch keine Bewertungen
- Photoelectric Effect: Dual Nature of Matter and RadiationsDokument21 SeitenPhotoelectric Effect: Dual Nature of Matter and RadiationsVishesh SheraNoch keine Bewertungen
- British International College: Year 11 Half Term Assessment ChemistryDokument9 SeitenBritish International College: Year 11 Half Term Assessment ChemistryHarry SonNoch keine Bewertungen
- Chemistry Form 4 Lesson 12Dokument8 SeitenChemistry Form 4 Lesson 12Sakinah SaadNoch keine Bewertungen
- Study of Beta Ray AbsorptionDokument4 SeitenStudy of Beta Ray AbsorptionWasimNoch keine Bewertungen
- Worksheet Elements Compounds Mixtures ks3Dokument4 SeitenWorksheet Elements Compounds Mixtures ks3eric sivanesh0% (1)
- Analytic ChemDokument316 SeitenAnalytic ChemoctavianistrateNoch keine Bewertungen
- Chapter 2 Chemical Context of LifeDokument8 SeitenChapter 2 Chemical Context of LifeJADEN MANNNoch keine Bewertungen
- Supplementary Problems 5.10 A Heat Pump ProvidesDokument3 SeitenSupplementary Problems 5.10 A Heat Pump Providesshodik8426134Noch keine Bewertungen
- Polystyrene Lab Report FinishedDokument2 SeitenPolystyrene Lab Report Finishedapi-510614531Noch keine Bewertungen
- ACBPD Lecture4 2017Dokument39 SeitenACBPD Lecture4 2017MohamedTaherNoch keine Bewertungen
- CH 5 Cls VIIA ChemistryDokument3 SeitenCH 5 Cls VIIA ChemistryTrixxy CarterNoch keine Bewertungen
- Lecture 35 PDFDokument20 SeitenLecture 35 PDFRachit ShahNoch keine Bewertungen
- Check List of Organic Chemistry Topics For B17CA and B17CB AppendedDokument6 SeitenCheck List of Organic Chemistry Topics For B17CA and B17CB AppendedSarah FeyNoch keine Bewertungen
- 10th Science Practice TestDokument13 Seiten10th Science Practice Testavinash960Noch keine Bewertungen
- PESSAT Chemistry Model PaperDokument12 SeitenPESSAT Chemistry Model PaperpullagalkNoch keine Bewertungen