Beruflich Dokumente
Kultur Dokumente
Acid - Base Titration: Reaction of Naoh With HCL
Hochgeladen von
Acamlee AriffinOriginalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Acid - Base Titration: Reaction of Naoh With HCL
Hochgeladen von
Acamlee AriffinCopyright:
Verfügbare Formate
1.
Acid - Base Titration:
Reaction of NaOH with HCl
Figure 1
Introduction
In aqueous solutions, addition of bases to water leads to an increase in the pH of the
solution, while the addition of acids leads to a decrease in the pH. The changes in the
pH can be followed using either specific dyes, called indicators, or a pH electrode. Acids
and bases neutralize, or reverse, the action of one another. By adding a known amount
Acid - Base Titration: Reaction of NaOH with HCl 5
of acid to a basic solution, until it completely reacts with it, the amount of the base can
be determined. This procedure is called: Acid - Base Titration. During neutralization,
acids and bases react with each other to produce ionic substances, called salts.
In this experiment, changes in pH and temperature, occurring while an acid (hydrochloric
acid) is added to a base (sodium hydroxide) solution, are followed using a pH electrode
and a Temperature sensor.
Equipment
• Nova5000
• A pH electrode
• A Temperature sensor (-25 °C to 110 °C)
• A polystyrene coffee cup
• 50 ml buret pipette
• A glass funnel
• 2 g NaOH
• 100 ml NHCl solution
• Magnetic stirrer
Equipment Setup Procedure
1. Launch MultiLab.
2. Connect the pH electrode to Input 1 (I/O-1) of the Nova5000.
3. Connect the Temperature sensor to Input 2 (I/O-2) of the Nova5000.
4. Assemble the equipment as illustrated in Figure 1.
5. Click Setup on the main toolbar and program the data logger according to the
setup specified below.
6 Acid - Base Titration: Reaction of NaOH with HCl
Data Logger Setup
Sensors:
Input 1: pH
Input 2: Temperature (-25 °C to 110 °C)
Rate:
Every second
Samples:
2000 samples
Experimental Procedure
1. Prepare a polystyrene cover for the polystyrene coffee cup. The cover should be flat
and larger than the circumference of the coffee cup (see Figure 1).
2. Pierce three holes in the cover: One for the pH electrode, one for the Temperature
sensor and one for the glass funnel.
3. Add 50 ml 0.5 N NaOH solution to the coffee cup.
4. Place the coffee cup on a magnetic stirrer.
5. Place the cover on the cup.
6. Click Run on the upper toolbar to begin recording data.
7. Wait until readings from the sensors are stable.
8. Start stirring the NaOH solution in the coffee cup.
9. Add, drop-by-drop, 1 N HCl solution from the buret to the cup, through the glass
funnel.
10. Follow changes in pH and temperature registered on the monitor.
11. As the pH just starts to change, stop the flow of HCl. Find the volume of HCl added
until that point.
12. Renew the flow of HCl. Follow pH changes very carefully.
13. Stop immediately the flow of HCl as the pH level stabilizes.
Acid - Base Titration: Reaction of NaOH with HCl 7
14. Save your data by clicking Save on the upper toolbar.
Data Analysis
1. Use the First cursor and Second cursor to display the change in the pH
obtained during the neutralization process.
What was the initial pH value? The final value? The difference between the two
values?
The Cursor: You can display up to two cursors on the graph simultaneously.
Use the first cursor to display individual data recording values, to select a curve or to
reveal the hidden Y-axis.
Use two cursors to display the difference between two coordinate values or to select a
range of data points.
To display the First cursor: Double click on an individual data point or click First cursor
on the graph toolbar. You can drag the cursor with the mouse onto any other point
on the plot, or onto a different plot. For finer cursor movements click Forward and
Backward cursor.
The coordinate values of the selected point will appear in the information bar at the
bottom of the graph window.
To display the Second cursor: Double click again anywhere on the graph area or click
Second cursor .
The information bar will now display the difference between the two coordinate values.
To remove the cursors: Click to remove the Second cursor and click to remove
the First cursor.
2. What was the volume of HCl added until the pH started to change? Compare it with
the volume of HCl added until a complete neutralization of NaOH was achieved.
3. Use the graph cursors to find the time interval between the point of start of change in
pH and the point of pH start of stabilization.
8 Acid - Base Titration: Reaction of NaOH with HCl
4. Use the graph cursors to display the change in temperature obtained in the process
and the time needed to reach the final temperature value.
5. Calculate the heat of reaction: C- water capacity, ΔT - temperature change.
Q = mcΔT
Note: Water specific heat capacity at 25 °C is 4.18 (J/g*°C).
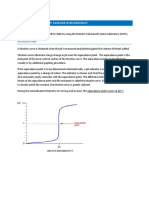
An example of the graph obtained in this experiment is shown below:
pH
Temp
Figure 2
Questions
1. Did you observe a fast pH change? Explain the difference in the short time interval
needed for the completion of the drastic changes of pH and that of the whole
neutralization process.
2. Is the neutralization reaction an exothermic reaction? Base your conclusions on the
experiment you performed.
3. Try to assume the results of acid - base reaction at different concentrations of NaOH
added to the coffee cup. What will be the pH change in each case? What will be the
extent of change in the temperature?
4. What will be the effect of reacting other acids (such as, for example, acetic acid) with
NaOH?
Acid - Base Titration: Reaction of NaOH with HCl 9
Further Suggestions
1. React different concentrations of NaOH with a constant concentration of HCl.
2. Calculate unknown concentrations of the titrated NaOH (or HCl). This can be
achieved by setting the flow of acid (or base) from the Buret pipette at a constant
rate (from the time interval on X axis multiplied by the flow rate, the volume added,
can be calculated).
3. Examine the effect of increasing the water and/or the surrounding temperature, on
the reaction.
4. Perform Acid - Base reaction with different types of acids and/or bases: A weak acid
with a strong base and vice versa.
10 Acid - Base Titration: Reaction of NaOH with HCl
Das könnte Ihnen auch gefallen
- Acid - Base Titration: Reaction of Naoh With HCL: ChemistryDokument6 SeitenAcid - Base Titration: Reaction of Naoh With HCL: ChemistryAchinthya PereraNoch keine Bewertungen
- Acid - Base Lab 2016 BegleyDokument7 SeitenAcid - Base Lab 2016 BegleyIsaac SnitkoffNoch keine Bewertungen
- Titration of A Diprotic Acid Identifying An Unknown: ObjectiveDokument9 SeitenTitration of A Diprotic Acid Identifying An Unknown: ObjectivePuji WulandariNoch keine Bewertungen
- Experiment 1&2Dokument8 SeitenExperiment 1&2Fatima AhmedNoch keine Bewertungen
- AP Chemistry - Titration Curves of Strong and Weak Acids and BasesDokument5 SeitenAP Chemistry - Titration Curves of Strong and Weak Acids and BasesJonathan Chen100% (2)
- PH Titration LabQuest PDFDokument3 SeitenPH Titration LabQuest PDFAntónio MatiasNoch keine Bewertungen
- Practical 4Dokument2 SeitenPractical 4vimukthi gunasinghaNoch keine Bewertungen
- Weak Acid Strong Base Titration LabDokument8 SeitenWeak Acid Strong Base Titration Labapi-265089380100% (1)
- 24 Acid-Base TitrationDokument5 Seiten24 Acid-Base Titrationgardarr11Noch keine Bewertungen
- GA7 Potentio Titr Rev7 99Dokument9 SeitenGA7 Potentio Titr Rev7 99Jerome SadudaquilNoch keine Bewertungen
- Sulfamic Acid Titration C12!5!10Dokument5 SeitenSulfamic Acid Titration C12!5!10Anonymous 1gXoNDYcNoch keine Bewertungen
- Chemistry Laboratory (CY1094D) : Dr. Mausumi ChattopadhyayaDokument18 SeitenChemistry Laboratory (CY1094D) : Dr. Mausumi ChattopadhyayaSita kumarNoch keine Bewertungen
- Acid-Base Titration by Dan HolmquistDokument5 SeitenAcid-Base Titration by Dan HolmquistPaul SchumannNoch keine Bewertungen
- Titration of A Diprotic Acid: Identifying An Unknown by Dan HolmquistDokument8 SeitenTitration of A Diprotic Acid: Identifying An Unknown by Dan HolmquistPaul Schumann0% (1)
- Lab R.2 - Concentration of An Acid - Three WaysDokument6 SeitenLab R.2 - Concentration of An Acid - Three WaysAdarsh Raj TiwariNoch keine Bewertungen
- Titration LabDokument8 SeitenTitration LabFarhan HabibzaiNoch keine Bewertungen
- PI Exp9 CompiledDokument12 SeitenPI Exp9 CompiledZaid SalmanNoch keine Bewertungen
- Titration Curves of Strong and Weak Acids and BasesDokument3 SeitenTitration Curves of Strong and Weak Acids and BasesMatthew Runyon50% (2)
- S Y PH MeterDokument2 SeitenS Y PH MeterNeelam KapoorNoch keine Bewertungen
- Experiment - 1: Determination of Strength of An Acid Using A PH MeterDokument16 SeitenExperiment - 1: Determination of Strength of An Acid Using A PH MeterAman Kumar0% (1)
- Neutralization ReactionsDokument3 SeitenNeutralization ReactionsShan Abi keash-1223Noch keine Bewertungen
- Heats of Reaction and Hess PDFDokument12 SeitenHeats of Reaction and Hess PDFs sNoch keine Bewertungen
- Isothermal Batch ReactorDokument10 SeitenIsothermal Batch ReactorSaswiny Ritchie0% (2)
- Potentiometric Titration CurvesDokument5 SeitenPotentiometric Titration CurvesDavid GrahamNoch keine Bewertungen
- Potentiometric Titration of Strong Acid With Strong Base: ExperimentDokument4 SeitenPotentiometric Titration of Strong Acid With Strong Base: ExperimentBasheer AhammadNoch keine Bewertungen
- Experiment 2Dokument6 SeitenExperiment 2Raj PatelNoch keine Bewertungen
- Applications of Lechats PrincipleDokument5 SeitenApplications of Lechats PrincipleBob BenburgNoch keine Bewertungen
- Experiment - H2SO4 Titration With NaOHDokument5 SeitenExperiment - H2SO4 Titration With NaOHfreeharshaNoch keine Bewertungen
- Part I: Titration With An Indicator: Data and ObservationsDokument5 SeitenPart I: Titration With An Indicator: Data and ObservationsjiNoch keine Bewertungen
- Lab ReportDokument7 SeitenLab ReportRakan DamasNoch keine Bewertungen
- Phosphoric Acid TitrationDokument3 SeitenPhosphoric Acid Titrationkarachi85Noch keine Bewertungen
- Potentiometric Titration Ex17Dokument10 SeitenPotentiometric Titration Ex17Tien HaminhNoch keine Bewertungen
- Determining The Enthalpy of A Chemical Reaction: ObjectivesDokument5 SeitenDetermining The Enthalpy of A Chemical Reaction: ObjectivesbooklookingboiNoch keine Bewertungen
- Chemistry Investigatory ProjectDokument11 SeitenChemistry Investigatory ProjectAryan WaratheNoch keine Bewertungen
- Exp2 - A-B Titration - w2013-2Dokument10 SeitenExp2 - A-B Titration - w2013-2lovingbubblesNoch keine Bewertungen
- Experiment 6 Titration II - Acid Dissociation ConstantDokument8 SeitenExperiment 6 Titration II - Acid Dissociation ConstantPanneer SelvamNoch keine Bewertungen
- Shayma Chem II Lab Manual.... Petrochemical Engineering DepartmentDokument55 SeitenShayma Chem II Lab Manual.... Petrochemical Engineering DepartmentMUHAMMAD AKRAM100% (1)
- Liquid Phase Chemical Reactor FinalDokument38 SeitenLiquid Phase Chemical Reactor FinalToMemNoch keine Bewertungen
- CHEM A 24 COMP Half TitrationDokument4 SeitenCHEM A 24 COMP Half TitrationSung Hoon ParkNoch keine Bewertungen
- Exp 1,2,3Dokument13 SeitenExp 1,2,3JWAN RA YA3QOBNoch keine Bewertungen
- Chem A 13 Comp EnthalpyDokument5 SeitenChem A 13 Comp EnthalpyKrystela Cariza Ramos MercadoNoch keine Bewertungen
- KaDokument5 SeitenKaSonu DubeyNoch keine Bewertungen
- Experiment PHDokument8 SeitenExperiment PHMohamed MubarakNoch keine Bewertungen
- Sample Chemistry Undergraduate Laboratory ReportDokument14 SeitenSample Chemistry Undergraduate Laboratory ReportApril TapayanNoch keine Bewertungen
- Experiment 3Dokument2 SeitenExperiment 3DARREN JOHN MUUWILNoch keine Bewertungen
- Enthalpy of Solution and ReactionDokument5 SeitenEnthalpy of Solution and ReactionCarmen GoguNoch keine Bewertungen
- Experiment 4 Preparation of Standardized SolutionsDokument10 SeitenExperiment 4 Preparation of Standardized SolutionsJohn Dy100% (1)
- CWV 24 COMP Acid - Base - Titration PDFDokument8 SeitenCWV 24 COMP Acid - Base - Titration PDFTha KantanaNoch keine Bewertungen
- Lebanese International University School of Pharmacy Pharmaceutical Sciences Department Pharmaceutical Analysis LaboratoryDokument9 SeitenLebanese International University School of Pharmacy Pharmaceutical Sciences Department Pharmaceutical Analysis LaboratoryRania ThiniNoch keine Bewertungen
- p4 En303 Manvi Nilaya 2k20en44Dokument8 Seitenp4 En303 Manvi Nilaya 2k20en442K20 EN 63 Sankalp PurwarNoch keine Bewertungen
- Determining The Enthalpy of A Chemical Reaction: ComputerDokument5 SeitenDetermining The Enthalpy of A Chemical Reaction: ComputerCristian AlamosNoch keine Bewertungen
- Lab Format:: Lab 2: Determination of Carbonate/BicarbonateDokument5 SeitenLab Format:: Lab 2: Determination of Carbonate/BicarbonateAnaya FatimaNoch keine Bewertungen
- Lab Report Acid BaseDokument4 SeitenLab Report Acid Basexuni34Noch keine Bewertungen
- AP Chemistry Investigation 4 - Judy, Paul, AnthonyDokument13 SeitenAP Chemistry Investigation 4 - Judy, Paul, AnthonyAnthony HowerNoch keine Bewertungen
- Buffers Lab Write UpDokument7 SeitenBuffers Lab Write Upapi-20078641850% (2)
- Thermometric Titrimetry: International Series of Monographs in Analytical ChemistryVon EverandThermometric Titrimetry: International Series of Monographs in Analytical ChemistryNoch keine Bewertungen
- Abridged Thermodynamic and Thermochemical Tables: SI UnitsVon EverandAbridged Thermodynamic and Thermochemical Tables: SI UnitsNoch keine Bewertungen
- Practice Makes Perfect in Chemistry: Kinetics and EquilibriumVon EverandPractice Makes Perfect in Chemistry: Kinetics and EquilibriumNoch keine Bewertungen
- Advanced Pharmaceutical analysisVon EverandAdvanced Pharmaceutical analysisBewertung: 4.5 von 5 Sternen4.5/5 (2)
- English Articles Korean ArticlesDokument31 SeitenEnglish Articles Korean ArticlesAcamlee AriffinNoch keine Bewertungen
- Marking Form Case StudyDokument2 SeitenMarking Form Case StudyAcamlee AriffinNoch keine Bewertungen
- Full ScheduleDokument1 SeiteFull ScheduleAcamlee AriffinNoch keine Bewertungen
- 23rd JULYDokument1 Seite23rd JULYAcamlee AriffinNoch keine Bewertungen
- Chem 28.1 E10 ATQDokument3 SeitenChem 28.1 E10 ATQSheenly Anne SaavedraNoch keine Bewertungen
- A1 Amino AcidsDokument6 SeitenA1 Amino AcidsGabby Tanaka0% (2)
- Experiment Chemistry 6.3 & 6.4 Form 4 KSSMDokument3 SeitenExperiment Chemistry 6.3 & 6.4 Form 4 KSSMNURNAIELA100% (1)
- Ionic EquilibriaDokument64 SeitenIonic EquilibriaArima KouseiNoch keine Bewertungen
- Chem 321 Exam 1 Study GuideDokument1 SeiteChem 321 Exam 1 Study Guideapi-245391028Noch keine Bewertungen
- Acid-Base Titration Using PH Meter and Finding The Equivalence Point Naoh ConcentrationDokument8 SeitenAcid-Base Titration Using PH Meter and Finding The Equivalence Point Naoh ConcentrationYocobSamandrewsNoch keine Bewertungen
- Some Very Important Value of Increasingand DecreasingDokument3 SeitenSome Very Important Value of Increasingand DecreasingGourab SahaNoch keine Bewertungen
- Lab ReportDokument8 SeitenLab ReportNAEEM MALIKNoch keine Bewertungen
- FC Assignment 07 Acid StrengthDokument4 SeitenFC Assignment 07 Acid StrengthHarsh Vardhan Singh100% (1)
- Biochemistry Harper's CH 2Dokument3 SeitenBiochemistry Harper's CH 2Ann Ross FernandezNoch keine Bewertungen
- Articulo Sobre La Determinación Experimental Del Ácido Fosfórico en La Coca ColaDokument5 SeitenArticulo Sobre La Determinación Experimental Del Ácido Fosfórico en La Coca ColaDiego Isac Cruz LopezNoch keine Bewertungen
- Alkalimetry in Alcohol-Water Mixtures With Potentiometric End-Point Detection Critical Remarks On A Newer Method ofDokument6 SeitenAlkalimetry in Alcohol-Water Mixtures With Potentiometric End-Point Detection Critical Remarks On A Newer Method ofErik AristyaNoch keine Bewertungen
- Notes - Pharmaceutical SaltsDokument27 SeitenNotes - Pharmaceutical SaltsTanaz NathaniNoch keine Bewertungen
- 4.3 HH EquationDokument34 Seiten4.3 HH EquationAdinda Nur AdilaNoch keine Bewertungen
- PH Curves & Indicators: Www. .CO - UKDokument23 SeitenPH Curves & Indicators: Www. .CO - UKJakeNoch keine Bewertungen
- The Ca2 EDTA Chelation As Standard Reaction To Validate Isothermal Titration Calorimeter Measurements Rafols Est Al 2016Dokument9 SeitenThe Ca2 EDTA Chelation As Standard Reaction To Validate Isothermal Titration Calorimeter Measurements Rafols Est Al 2016Ariel CondoriNoch keine Bewertungen
- 102 AbrevsDokument7 Seiten102 AbrevsHandugan Quinlog NoelNoch keine Bewertungen
- Chemical Engineering Lab ReportDokument7 SeitenChemical Engineering Lab ReportNazario Emil LintagNoch keine Bewertungen
- T8 Titration CalculationsDokument1 SeiteT8 Titration CalculationsMarcus WrightNoch keine Bewertungen
- Amina PrimerDokument13 SeitenAmina PrimerYoan YasrilNoch keine Bewertungen
- Gen Chem Practice Problems Ch10, 18 & Buffers f08Dokument6 SeitenGen Chem Practice Problems Ch10, 18 & Buffers f08Anonymous rFIshYyNoch keine Bewertungen
- Carboxylic Acids and Their SaltsDokument2 SeitenCarboxylic Acids and Their SaltsfaithNoch keine Bewertungen
- Past Paper Topic 8 9Dokument29 SeitenPast Paper Topic 8 911035030Noch keine Bewertungen
- Sodamint PDFDokument5 SeitenSodamint PDFalwi firdausNoch keine Bewertungen
- Clinical Chemistry II ELECTROLYTESDokument2 SeitenClinical Chemistry II ELECTROLYTESEden MaeNoch keine Bewertungen
- Acid Base Equilibria PDFDokument30 SeitenAcid Base Equilibria PDFLin Xian XingNoch keine Bewertungen
- Lab. 6 Buffer SolutionsDokument4 SeitenLab. 6 Buffer Solutionssultan100% (1)
- Buffer (Larutan Penyangga)Dokument6 SeitenBuffer (Larutan Penyangga)Budiman ApriyossaNoch keine Bewertungen
- Neutralization Reaction: Determine PH of Acid-Base MixtureDokument12 SeitenNeutralization Reaction: Determine PH of Acid-Base MixtureHasan BusriNoch keine Bewertungen
- Justine Chirichella - Lab 3 BiochemistryDokument7 SeitenJustine Chirichella - Lab 3 Biochemistryapi-310022767Noch keine Bewertungen