Beruflich Dokumente
Kultur Dokumente
CH16 Aqueous Equilibria
Hochgeladen von
Carlos Mella-RijoOriginalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
CH16 Aqueous Equilibria
Hochgeladen von
Carlos Mella-RijoCopyright:
Verfügbare Formate
CHEM 1442
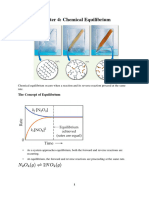
Chapter 16: Aqueous Equilibria
16.1 The Common Ion Effect
Common Ion- an ion in solution that comes from two different sources.
The Common Ion Effect- the presence of a common ion represses ionization of a weak electrolyte. It is
an application of Le Chatelier’s Principle.
16.2 Buffer Solutions
Buffer Solution- a solution that resists changes in pH when a small amount of an acid or base is added.
o A buffer solution contains a weak conjugate acid-base pair, such as HA and A-.
If a strong acid is added to the solution, the weak base consumes it.
If a strong base is added, the weak acid consumes it.
o There are three ways that buffers can be prepared:
By mixing a weak acid or base with its salt.
By mixing a weak acid with approximately half as much strong base.
By mixing a weak base with approximately half as much strong acid.
The pH of a buffer can be determined with the Henderson-Hasselbalch Equation:
[base ]
pH= p K a + log
[acid]
Buffer Capacity- the amount of acid or base that can be added to a buffer before its buffering ability is
degraded.
o Buffer Range is plus or minus one pH units from the pKa value.
16.3 Acid-Base Titrations
An acid-base titration is a neutralization reaction, where an acid and base react to form a salt and water.
In a titration, either the acid or the base is a standardized solution, which is the solution with a known
concentration. This can be used to find the concentration of the other solution.
Remember the titration terms:
o Titrant- a solution of known concentration added to the analyte.
o Analyte- the substance with unknown concentration.
o Equivalence Point- point when the moles of titrant added and the moles of analyte are
chemically equivalent.
o Endpoint- the point when a reaction is assumed complete, usually shown by an indicator.
The pH at any point in a titration can be determined by writing the chemical equation for the reaction
that occurs and then using a BCA table to determine the quantities remaining after the reaction. This
is then followed by an equilibrium calculation when weak species are present.
A pH curve is a plot of the solution as a function of the amount of titrant added.
16.4 The Solubility Product Constant ( K ) sp
Sparingly Soluble Salts- ionic compounds with low solubilities, which were classified as insoluble in
chapter 4.
o Only a very small amount of a sparingly soluble salt saturates their solutions in water.
o Like in all solutions, the salts then exist in equilibrium between undissolved solid and their
dissociated ions in the solution.
Their equilibrium constant is called the solubility-product constant, K sp .
It is calculated from the product of the concentrations of the dissociated ions in
the solution to the power of their respective coefficients (since the only
reactants are the solid salt and liquid water, both of which are omitted from
equilibrium constant calculations.)
The molar solubility( Sm) of a substance is it’s solubility expressed in Molarity. Can be found through an
ICE table.
o K sp can be found from experimental data. When K sp is known, we can find Sm, both in pure
water and in the presence of a common ion.
The solubility of many sparingly soluble salts changes with pH. In order to determine if the pH of the
solution will affect solubility, focus on the anion:
o If the anion comes from a strong acid, it is a spectator ion in acid-base reactions.
o If the anion is the conjugate base of a weak acid, the salt will become more soluble as pH is
lowered.
o If two solutions containing the ions of a sparingly soluble salt are mixed, the Q sp value of the
mixture can be compared to K sp to determine if precipitation will occur.
If Q sp < K sp, the solution is unsaturated, and no precipitation occurs.
If Q sp > K sp,the is supersaturated, and precipitation occurs.
If Q sp=K sp, the solution is saturated, and no net change occurs.
Das könnte Ihnen auch gefallen
- Jack Westin MCAT Content General ChemistryDokument25 SeitenJack Westin MCAT Content General ChemistryLora100% (1)
- Solutions Constant of A Weak Acid or BaseDokument8 SeitenSolutions Constant of A Weak Acid or BaseJeromeNoch keine Bewertungen
- Scientific Method: Jayson Nota Maria Angelica BalicocoDokument21 SeitenScientific Method: Jayson Nota Maria Angelica BalicocoGrace Ann ArimadoNoch keine Bewertungen
- Unit 4 Chemical Equilibrium PDFDokument27 SeitenUnit 4 Chemical Equilibrium PDFmisganamarcos10Noch keine Bewertungen
- Weak Acid Vs Strong Base TitrationDokument9 SeitenWeak Acid Vs Strong Base Titrationgovindshankarq9Noch keine Bewertungen
- Acid and BasesDokument2 SeitenAcid and Basesibbie133Noch keine Bewertungen
- 2.pH, Buffers and IsotonicDokument48 Seiten2.pH, Buffers and Isotonicrajender91% (11)
- SOURCE: General Chemistry: Principles and Modern Applications 10Dokument3 SeitenSOURCE: General Chemistry: Principles and Modern Applications 10Jerremiah YuNoch keine Bewertungen
- 2.0 Literature Review 2.1 PH: PH Log pOH Log PH 14 pOHDokument13 Seiten2.0 Literature Review 2.1 PH: PH Log pOH Log PH 14 pOHNorzulaika AmitNoch keine Bewertungen
- 6 - IonizationDokument48 Seiten6 - IonizationYashfa YasinNoch keine Bewertungen
- Physical Pharmacy: Lec 8 DR Basam Al ZayadyDokument6 SeitenPhysical Pharmacy: Lec 8 DR Basam Al ZayadyVikash KushwahaNoch keine Bewertungen
- Physical Pharmacy 4Dokument11 SeitenPhysical Pharmacy 4husseinNoch keine Bewertungen
- Chem1101 - Acid, Bases, and Aqueous EquilibriumDokument19 SeitenChem1101 - Acid, Bases, and Aqueous EquilibriumCharlotte HooperNoch keine Bewertungen
- Edexcel Chemistry A-Level: Topic 12: Acid-Base EquilibriaDokument11 SeitenEdexcel Chemistry A-Level: Topic 12: Acid-Base EquilibriaLulwa KhaskiehNoch keine Bewertungen
- PH and BufferDokument31 SeitenPH and Bufferhelion45Noch keine Bewertungen
- Acid-Base Equilibria in Aqueous SolutionsDokument48 SeitenAcid-Base Equilibria in Aqueous SolutionsAdrian ChombaNoch keine Bewertungen
- Elements of TitrationDokument4 SeitenElements of TitrationChyna M. MangibinNoch keine Bewertungen
- The Ka and KB of ItDokument23 SeitenThe Ka and KB of ItAditya VermaNoch keine Bewertungen
- Quantitative Acid Equilibrium Constant Dissociation Acid-Base Reactions Acid Conjugate Base Hydrogen Ion Proton Hydronium IonDokument2 SeitenQuantitative Acid Equilibrium Constant Dissociation Acid-Base Reactions Acid Conjugate Base Hydrogen Ion Proton Hydronium IonamirahrazaliNoch keine Bewertungen
- Acid Base TheoriesDokument46 SeitenAcid Base TheoriesAltamash KhanNoch keine Bewertungen
- 19 Reactions of Acids and BasesDokument19 Seiten19 Reactions of Acids and BasesrachelelizabethNoch keine Bewertungen
- Chem 142 - N Lab Acid Base Equilibria and Buffer Solutions 2022Dokument64 SeitenChem 142 - N Lab Acid Base Equilibria and Buffer Solutions 2022Jahred CantornaNoch keine Bewertungen
- Topic 8 - Acids and BasesDokument5 SeitenTopic 8 - Acids and BasesRudy YoishoNoch keine Bewertungen
- Equilibria (Chapter 7) : PH Is Defined As The Negative Logarithm To The Base 10 of The Hydrogen Ion ConcentrationDokument9 SeitenEquilibria (Chapter 7) : PH Is Defined As The Negative Logarithm To The Base 10 of The Hydrogen Ion ConcentrationumerNoch keine Bewertungen
- Citric AcidDokument1 SeiteCitric Acidahmad faisal rosidiNoch keine Bewertungen
- Acid Base Titrations 11II PDFDokument35 SeitenAcid Base Titrations 11II PDFŠĭlệncěIšmyPŕIdệNoch keine Bewertungen
- PH and BufferDokument3 SeitenPH and BufferMuhammad YaseenNoch keine Bewertungen
- Reading Material For Experiment 3Dokument4 SeitenReading Material For Experiment 3Abdallah AlhasanNoch keine Bewertungen
- Equilibrium Constants: Needed in ChemistryDokument28 SeitenEquilibrium Constants: Needed in ChemistryGea EcoyNoch keine Bewertungen
- Wiki Anal Tit Nonaqueous TitrationDokument5 SeitenWiki Anal Tit Nonaqueous TitrationMira JunitaNoch keine Bewertungen
- 2.pH, Buffers and Isotonic Solutions AbDokument48 Seiten2.pH, Buffers and Isotonic Solutions AbPasham Venkat ReddyNoch keine Bewertungen
- Buffer Solution (1.2)Dokument5 SeitenBuffer Solution (1.2)Kuldipsinh ZalaNoch keine Bewertungen
- PH METERS, HYDROLYSIS, AND BUFFER CAPACITYDokument10 SeitenPH METERS, HYDROLYSIS, AND BUFFER CAPACITYnermeen ahmedNoch keine Bewertungen
- Name: Yambesa Surname: Mphathiswa STUDENT NUMBER: 202250111 Group D1 Experiment 3Dokument10 SeitenName: Yambesa Surname: Mphathiswa STUDENT NUMBER: 202250111 Group D1 Experiment 3YAMBESA MphathiswaNoch keine Bewertungen
- Acid Base Titration: Ha + H O H O + A (Acid) B O BH + Oh (Base)Dokument6 SeitenAcid Base Titration: Ha + H O H O + A (Acid) B O BH + Oh (Base)Ben AbellaNoch keine Bewertungen
- General Chemistry 2 (STEM) : Quarter 4 - Module 3 & 4Dokument9 SeitenGeneral Chemistry 2 (STEM) : Quarter 4 - Module 3 & 4Alexa ValdezNoch keine Bewertungen
- Analytical Chemistry (Volumetric Analysis)Dokument25 SeitenAnalytical Chemistry (Volumetric Analysis)OMED gardiNoch keine Bewertungen
- SolutionDokument14 SeitenSolutionAstuti GendaNoch keine Bewertungen
- CN 3Dokument32 SeitenCN 3Michelle Dela CruzNoch keine Bewertungen
- Acid-Base Equilibria and Solubility EquilibriaDokument24 SeitenAcid-Base Equilibria and Solubility EquilibriaAndrew John CellonaNoch keine Bewertungen
- Edexcel IAL Chemistry A-Level: Topic 14: Acid-Base EquilibriaDokument11 SeitenEdexcel IAL Chemistry A-Level: Topic 14: Acid-Base EquilibriaMer CyNoch keine Bewertungen
- HENDERSON HASSLE BACH EQUATION FinalDokument39 SeitenHENDERSON HASSLE BACH EQUATION FinalMaria khurshidNoch keine Bewertungen
- CHEM 102 Instructional Objectives: - Additional Aqueous EquilibriaDokument29 SeitenCHEM 102 Instructional Objectives: - Additional Aqueous EquilibriarajNoch keine Bewertungen
- Acid-Base EquilibriumDokument54 SeitenAcid-Base EquilibriumFloralba ZapataNoch keine Bewertungen
- Lec 7 Analytical (Buffer Solution)Dokument13 SeitenLec 7 Analytical (Buffer Solution)alhyaly857Noch keine Bewertungen
- Basic Pharmaceutical Chemistry 15Dokument108 SeitenBasic Pharmaceutical Chemistry 15Gideon AntwiNoch keine Bewertungen
- Buffers CompleteDokument46 SeitenBuffers CompleteSunshine_Bacla_4275100% (2)
- Acid and Salt EquilibriaDokument26 SeitenAcid and Salt EquilibriaAnthony AbesadoNoch keine Bewertungen
- Lecture 1Dokument24 SeitenLecture 1mvps9248Noch keine Bewertungen
- Buffer Solutions.: Ass. Prof. I. R. BekusDokument27 SeitenBuffer Solutions.: Ass. Prof. I. R. BekusNanda ThyarezaNoch keine Bewertungen
- 14 Aqueous EquilibriaDokument39 Seiten14 Aqueous EquilibriaSheila PratiwiNoch keine Bewertungen
- Chapter 17: Additional Aspects of Aqueous Equilibria: Common-Ion EffectDokument28 SeitenChapter 17: Additional Aspects of Aqueous Equilibria: Common-Ion EffectrajNoch keine Bewertungen
- Waleeed Waleed BiochemistryDokument8 SeitenWaleeed Waleed Biochemistryali mughalNoch keine Bewertungen
- Assignment 2Dokument3 SeitenAssignment 2edelyn telewikNoch keine Bewertungen
- 줌달 10판 솔루션 (15-19)Dokument201 Seiten줌달 10판 솔루션 (15-19)tempusNoch keine Bewertungen
- Liat Rumusnya Di Ebook Halaman 677Dokument2 SeitenLiat Rumusnya Di Ebook Halaman 677Julio DavidNoch keine Bewertungen
- Acids and BasesRLLLLDokument7 SeitenAcids and BasesRLLLLThea GermanNoch keine Bewertungen
- Analytical Chemistry Acid-Base Titration: H) Ba (OH) H)Dokument5 SeitenAnalytical Chemistry Acid-Base Titration: H) Ba (OH) H)Samra ButtNoch keine Bewertungen
- Buffers and Salts Hydrolysis: 3-1. Preparation of Ammonium BufferDokument3 SeitenBuffers and Salts Hydrolysis: 3-1. Preparation of Ammonium BufferaisarachemNoch keine Bewertungen
- Advanced Pharmaceutical analysisVon EverandAdvanced Pharmaceutical analysisBewertung: 4.5 von 5 Sternen4.5/5 (2)
- Raytheon UAS Showcase - EE Team Notebook PDFDokument152 SeitenRaytheon UAS Showcase - EE Team Notebook PDFCarlos Mella-RijoNoch keine Bewertungen
- Chapter 12: Solutions: 12.1 Setting The Stage: Some Important TerminologyDokument5 SeitenChapter 12: Solutions: 12.1 Setting The Stage: Some Important TerminologyCarlos Mella-RijoNoch keine Bewertungen
- CH11 Intermolecular ForcesDokument5 SeitenCH11 Intermolecular ForcesCarlos Mella-RijoNoch keine Bewertungen
- Chapter 19: Electrochemistry: 19.1 Voltaic CellsDokument4 SeitenChapter 19: Electrochemistry: 19.1 Voltaic CellsCarlos Mella-RijoNoch keine Bewertungen
- Chapter 17: Chemical Thermodynamics: 17.1 When Is A Process Spontaneous?Dokument4 SeitenChapter 17: Chemical Thermodynamics: 17.1 When Is A Process Spontaneous?Carlos Mella-RijoNoch keine Bewertungen
- CH18 Oxidation-Reduction ReactionsDokument2 SeitenCH18 Oxidation-Reduction ReactionsCarlos Mella-RijoNoch keine Bewertungen
- Famous Bombers of The Second World War - 1st SeriesDokument142 SeitenFamous Bombers of The Second World War - 1st Seriesgunfighter29100% (1)
- Lesson PlanDokument18 SeitenLesson PlanYasmin Abigail AseriosNoch keine Bewertungen
- IDRW MagazineDokument10 SeitenIDRW MagazineVirarya100% (1)
- ESM-4810A1 Energy Storage Module User ManualDokument31 SeitenESM-4810A1 Energy Storage Module User ManualOscar SosaNoch keine Bewertungen
- Nurtured Womb e BookDokument22 SeitenNurtured Womb e BookSteph's Desserts100% (1)
- Ield Methods: A Typical Field Mapping Camp in The 1950sDokument4 SeitenIeld Methods: A Typical Field Mapping Camp in The 1950sshivam soniNoch keine Bewertungen
- MSDS PaintingDokument81 SeitenMSDS PaintingPaulina PaskahriaNoch keine Bewertungen
- Kulkarni Shilpa A.Dokument148 SeitenKulkarni Shilpa A.MSKCNoch keine Bewertungen
- Battery Installation ProcedureDokument5 SeitenBattery Installation ProceduresantoshkumarNoch keine Bewertungen
- Biasing Opamps Into Class ADokument11 SeitenBiasing Opamps Into Class AsddfsdcascNoch keine Bewertungen
- Bitsat Paper 5Dokument19 SeitenBitsat Paper 5pranka5240100% (1)
- DEAD STARS by Paz Marquez BenitezDokument17 SeitenDEAD STARS by Paz Marquez BenitezArmiethazen Khea Page PalarcaNoch keine Bewertungen
- Mid Lesson 1 Ethics & Moral PhiloDokument13 SeitenMid Lesson 1 Ethics & Moral PhiloKate EvangelistaNoch keine Bewertungen
- Curriculum VitaeDokument7 SeitenCurriculum VitaeRossy Del ValleNoch keine Bewertungen
- Mono 108Dokument438 SeitenMono 108pasaricaNoch keine Bewertungen
- Rumah Cerdas Bahasa Inggris Belajar Bahasa Inggris Dari Nol 4 Minggu Langsung BisaDokument3 SeitenRumah Cerdas Bahasa Inggris Belajar Bahasa Inggris Dari Nol 4 Minggu Langsung BisaArditya CitraNoch keine Bewertungen
- Lesson Notes Lecture 14Dokument5 SeitenLesson Notes Lecture 14Quantum SaudiNoch keine Bewertungen
- The Hollow Boy Excerpt PDFDokument52 SeitenThe Hollow Boy Excerpt PDFCathy Mars100% (1)
- CSEC Chemistry June 2018 P2 AnswersDokument7 SeitenCSEC Chemistry June 2018 P2 AnswerscxcchemistryNoch keine Bewertungen
- Introduction: Science and Environment: Brgy - Pampang, Angeles City, PhilippinesDokument65 SeitenIntroduction: Science and Environment: Brgy - Pampang, Angeles City, PhilippinesLance AustriaNoch keine Bewertungen
- The Royal Commonwealth Society of Malaysia: Function MenuDokument3 SeitenThe Royal Commonwealth Society of Malaysia: Function MenuMynak KrishnaNoch keine Bewertungen
- Chapter 01Dokument16 SeitenChapter 01deepak_baidNoch keine Bewertungen
- OLFACTIVE TRAINING 101 by SozioDokument36 SeitenOLFACTIVE TRAINING 101 by SoziojaviercdeaeNoch keine Bewertungen
- 基礎居合講座Dokument33 Seiten基礎居合講座任平生100% (1)
- Pediatric Gynecology BaruDokument79 SeitenPediatric Gynecology BaruJosephine Irena100% (2)
- ADC ManualDokument47 SeitenADC ManualRavi ShuklaNoch keine Bewertungen
- Science7 q2 Mod6of8 Asexual Sexualrep v2Dokument26 SeitenScience7 q2 Mod6of8 Asexual Sexualrep v2Ishi OcheaNoch keine Bewertungen
- Temposonics: Absolute, Non-Contact Position SensorsDokument23 SeitenTemposonics: Absolute, Non-Contact Position Sensorssorangel_123Noch keine Bewertungen
- Digital ElectronicsDokument18 SeitenDigital ElectronicsHarry BeggyNoch keine Bewertungen
- ForewordDokument96 SeitenForewordkkcmNoch keine Bewertungen