Beruflich Dokumente
Kultur Dokumente
GAS ABSORPTION - Report
Hochgeladen von
gzairene87620 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
3K Ansichten6 SeitenThe operation of absorption is applied in industry to purify process streams. It is used extensively to remove toxic or noxious components (pollutants) from effluent gas streams. There is a mass transfer of the component of the gas from the gas phase to the liquid phase.
Originalbeschreibung:
Originaltitel
GAS ABSORPTION - report
Copyright
© Attribution Non-Commercial (BY-NC)
Verfügbare Formate
DOC, PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenThe operation of absorption is applied in industry to purify process streams. It is used extensively to remove toxic or noxious components (pollutants) from effluent gas streams. There is a mass transfer of the component of the gas from the gas phase to the liquid phase.
Copyright:
Attribution Non-Commercial (BY-NC)
Verfügbare Formate
Als DOC, PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
3K Ansichten6 SeitenGAS ABSORPTION - Report
Hochgeladen von
gzairene8762The operation of absorption is applied in industry to purify process streams. It is used extensively to remove toxic or noxious components (pollutants) from effluent gas streams. There is a mass transfer of the component of the gas from the gas phase to the liquid phase.
Copyright:
Attribution Non-Commercial (BY-NC)
Verfügbare Formate
Als DOC, PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 6
GAS ABSORPTION
● also known as scrubbing
● an operation in which a gas mixture is contacted with a liquid
for the purpose of preferentially dissolving one or more components of
the gas mixture and to provide a solution of them in the liquid.
● there is a mass transfer of the component of the gas from the
gas phase to the liquid phase.
● the operation of absorption is applied in industry to purify
process streams or recover valuable components of the stream. It is
used extensively to remove toxic or noxious components (pollutants)
from effluent gas streams.
The absorption process requires the following steps:
1. diffusion of the solute gas molecules through the host gas to the
liquid boundary layer based on a concentration gradient
2. solvation of the solute gas in the host liquid based on gas-liquid
solubility
3. diffusion of the solute gas based on concentration gradient, thus
depleting the liquid boundary layer and permitting further solvation.
Method of Operation:
A. Counter-current Operation
● it was widely used in the industry.
● the gas enters the column or tower from below as leaves at the top,
while liquid enters from the top and flows in opposite direction and
exits from the bottom.
Figure for Dilute System:
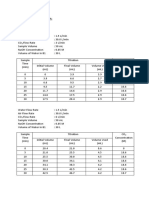
Notations : In terms of mole fraction and total flowrates
y : mole fraction of solute A in the gas phase
x : mole fraction of solute A in the liquid phase
G : total molar flowrate of the gas stream (gas flux), kg-moles/m2.s
L : total molar flowrate of the liquid stream, kg-moles/m2.s
Inside the column, mass transfer takes place as the solute
(component A) is absorbed by the liquid. The quantities of L and x (for
the liquid side) and G and y (for the gas side) varies continuously: as
we gradually move up the column, component A is continuously being
transferred from the gas phase to the liquid phase. Thus, in going up
the column, there is a decrease in the total gas flowrate, and a
decrease in the concentration of A in the gas phase. At the same time,
in going down the column, there is an increase in the total liquid
flowrate, and an increase in the concentration of A in the liquid phase.
Thus,
G1 > G > G 2 L1 > L > L 2
y1 > y > y 2 x1 > x > x 2
The relationship between these variables L, x, G and y is the
operating line equation. The operating line equation is obtained by
material balance around the column.
At steady-state: IN = OUT
Thus, G y + L 1 x1 = L x + G1 y1
Using the dilute system assumptions, we simply the equation and
obtain:
G y = L x + G y1 - L x1
Re-arranging:
Y =[ ] x +[
Since L and G are assumed to be approximately constant, the
operating line is a straight line of the form y = mx + c, with the
gradient of L / G, the liquid-to-gas ratio.
The operating line connects the 2 end points - point 1 (x1 , y1) that
represents conditions at the bottom of the column, and point 2 (x2 ,
y2) that represents conditions at the top of the column.
B. Co-current Gas Absorption
● This mode of operation is seldom used in practice.
● It is less efficient than counter-current operation.
The main points to note about this operation are as follow:
● The operating line has negative slope.
● The is no minimum liquid-to-gas ratio.
● To produce an exit liquid and gas streams at equilibrium (xe,
ye) on the equilibrium curve, an infinitely tall column must be used.
ABSORPTION EQUIPMENT
● Tray (or plate or stage) Columns - The types of trays used in
absorption include: sieve tray, valve tray and bubble-cap trays. These
internals are the same as those covered in "Distillation".
● Packed Column - Both random and structured packings had been
used.
● Spray Column - The gas flows upward continuously through an
open chamber in which scrubbing liquid droplets falls from spray
nozzles through the gas. The gas pressure drop is small, but separation
is not as good as the bubble column. This column is widely used for its
simplicity, low pressure drop, and resistance to scale deposition and
plugging.
● Bubble Column - The gas is forced under pressure through
perforated pipes submerged in the scrubbing liquid. As such the gas
phase is dispersed and the liquid phase is continuous. As the bubbles
rise through the liquid, absorption of the gas occurs. This type of
device suffers from the high pressure drop due to the liquid hydrostatic
head.
Types of Absorption Processes:
Physical Absorption
● can be used as an alternative to distillation for the separation
of light gases.
Chemical Absorption
● is important for removal of acid gases and for chemicals
production.
Application of Gas Absorption
1. Coking
2. Coal-Fired power plant
3. Natural Gas Production
4. Microelectronics Manufacturing
5. Ground Water Remediation
GAS DESORPTION
● also known as Stripping
● The process of removing gas dissolved in a liquid and where the liberated gas
is taken up in a gas or vapor in contact with the liquid.
● The mass transfer is in the opposite direction, i.e. from the
liquid phase to the gas phase.
Das könnte Ihnen auch gefallen
- Fluidization: Chemical Engneering PracticeDokument24 SeitenFluidization: Chemical Engneering PracticeFarhan M JafrI100% (1)
- Optimal cleaning cycle heat exchanger foulingDokument1 SeiteOptimal cleaning cycle heat exchanger foulingBahadır KayaNoch keine Bewertungen
- 4.3. Consider The Flowsheet For The Manufacture of Vinyl Chloride in Figure 4.8Dokument2 Seiten4.3. Consider The Flowsheet For The Manufacture of Vinyl Chloride in Figure 4.8Anonymous QwUTQlAO100% (1)
- Continuous Stirred Tank Reactor ExperimentDokument25 SeitenContinuous Stirred Tank Reactor ExperimentChristopher Emeka Ominyi100% (1)
- Mass Transfer 16CE2004 - HandoutDokument80 SeitenMass Transfer 16CE2004 - HandoutjAYNoch keine Bewertungen
- Absorption of Carbon Dioxide Into WaterDokument11 SeitenAbsorption of Carbon Dioxide Into WaterEstelle Jean CauilanNoch keine Bewertungen
- Effux Time EquationsDokument7 SeitenEffux Time EquationsDhananjay KadamNoch keine Bewertungen
- Modeling in Transport Phenomena: A Conceptual ApproachVon EverandModeling in Transport Phenomena: A Conceptual ApproachBewertung: 3 von 5 Sternen3/5 (2)
- Gas Absorption and Gas StrippingDokument14 SeitenGas Absorption and Gas StrippingEK63Noch keine Bewertungen
- Gas Absorption Process FundamentalsDokument34 SeitenGas Absorption Process Fundamentalslickaa200100% (1)
- Gas Absorption Theory, Apparatus, ProcedureDokument16 SeitenGas Absorption Theory, Apparatus, Proceduresolehah misni100% (1)
- AbsorptionDokument54 SeitenAbsorptionBebo El MasryNoch keine Bewertungen
- Introduction to Gas Absorption ProcessDokument18 SeitenIntroduction to Gas Absorption ProcessRanbir NarainNoch keine Bewertungen
- High-Pressure Fluid Phase Equilibria: Phenomenology and ComputationVon EverandHigh-Pressure Fluid Phase Equilibria: Phenomenology and ComputationNoch keine Bewertungen
- Gas AbsorptionDokument19 SeitenGas AbsorptionAnonymous NyvKBW100% (3)
- Liquid LiquidDokument8 SeitenLiquid LiquidAnonymous b9fcR5Noch keine Bewertungen
- Equilibrium Separation Operations: Lecture 1: IntroductionDokument23 SeitenEquilibrium Separation Operations: Lecture 1: IntroductionkaleijaNoch keine Bewertungen
- MSOCHA3 Tutorial 1 Multicomponent AbsorptionDokument5 SeitenMSOCHA3 Tutorial 1 Multicomponent AbsorptionTshwarelo MahlakoaneNoch keine Bewertungen
- Gas Diffusion UnitDokument20 SeitenGas Diffusion Unitsolehah misniNoch keine Bewertungen
- Gas Absorption LabDokument8 SeitenGas Absorption Labsolehah misni100% (1)
- Sample Problem #14Dokument7 SeitenSample Problem #14DozdiNoch keine Bewertungen
- 04 Script Examples Solid Liquid ExtractionDokument11 Seiten04 Script Examples Solid Liquid ExtractionLei YinNoch keine Bewertungen
- Unit Operation 1: CHE 321 (3 Units)Dokument70 SeitenUnit Operation 1: CHE 321 (3 Units)Glory UsoroNoch keine Bewertungen
- Week03 Multi Component Flash DistillationDokument31 SeitenWeek03 Multi Component Flash DistillationTirapath Chobchuen100% (1)
- C4 Lab ReportDokument11 SeitenC4 Lab ReportchaitanyaNoch keine Bewertungen
- Diffusivity of Liquid Into LiquidDokument8 SeitenDiffusivity of Liquid Into LiquidZahraa GhanemNoch keine Bewertungen
- Liquid Liquid Extraction ExperimentDokument17 SeitenLiquid Liquid Extraction Experimentmhd badhrul bin baharNoch keine Bewertungen
- Unit Operation Laboratory 2 (CCB 3062)Dokument7 SeitenUnit Operation Laboratory 2 (CCB 3062)Carl Erickson100% (1)
- CONTINUOUS DistillationDokument5 SeitenCONTINUOUS DistillationNaseer SattarNoch keine Bewertungen
- Absorption and StrippingDokument60 SeitenAbsorption and StrippingMyvizhi Somasundaram100% (2)
- Gas AbsorptionDokument24 SeitenGas AbsorptionShalini Krishnan100% (1)
- Solved Problem Question (Gas Ab)Dokument2 SeitenSolved Problem Question (Gas Ab)Seruzna IshxNoch keine Bewertungen
- CHE504 - Lab Report On Distillation ColuDokument27 SeitenCHE504 - Lab Report On Distillation ColuMuhammad Irfan MalikNoch keine Bewertungen
- Tutorial Reactive SystemsDokument33 SeitenTutorial Reactive Systemssiti azilaNoch keine Bewertungen
- TareaDokument3 SeitenTareaAydee GarciaNoch keine Bewertungen
- Lab 1Dokument12 SeitenLab 1JoeJeanNoch keine Bewertungen
- Gas AbsorptionDokument43 SeitenGas AbsorptionJoel Ong0% (1)
- Principles of AbsorptionDokument7 SeitenPrinciples of AbsorptionleeneotrillanesNoch keine Bewertungen
- Distillation Example 4 and 5Dokument2 SeitenDistillation Example 4 and 5DirkMyburghNoch keine Bewertungen
- Tutorial Leaching 2017Dokument11 SeitenTutorial Leaching 2017Victor M. Jaki100% (1)
- Vapor Liquid EquilibriumDokument28 SeitenVapor Liquid EquilibriumKhloud MadihNoch keine Bewertungen
- Series and Parallel Pumps: Flow Rate & PressureDokument11 SeitenSeries and Parallel Pumps: Flow Rate & PressureKevin Devastian100% (1)
- Packed Distillation Column ExperimentDokument20 SeitenPacked Distillation Column ExperimentChan Chun ChenNoch keine Bewertungen
- RI Vs Composition Methanol-Water MixtureDokument12 SeitenRI Vs Composition Methanol-Water MixtureAnonymous VeJYFSMWLINoch keine Bewertungen
- Azeotropic Mass BalanceDokument25 SeitenAzeotropic Mass BalancesowjanyaavkNoch keine Bewertungen
- Separation Processes - I (CHE F244) Total Marks - 15 Due Date & Time: 01/07/2020, 5:00 PM AssignmentDokument4 SeitenSeparation Processes - I (CHE F244) Total Marks - 15 Due Date & Time: 01/07/2020, 5:00 PM AssignmentElliot AldersonNoch keine Bewertungen
- Gas Absorption Lab ReportDokument3 SeitenGas Absorption Lab ReportNur Shaffikha Azmi100% (1)
- Optimal Operation of A Semi-Batch Reactive Distillation Column (2000)Dokument7 SeitenOptimal Operation of A Semi-Batch Reactive Distillation Column (2000)GodofredoNoch keine Bewertungen
- Humidification and Air Conditioning: Lecture No. 8Dokument6 SeitenHumidification and Air Conditioning: Lecture No. 8Anonymous UFa1z9XUANoch keine Bewertungen
- Gas Absorption: Determining Drag and Flooding FlowsDokument5 SeitenGas Absorption: Determining Drag and Flooding FlowsDean Joyce AlborotoNoch keine Bewertungen
- Cet Mkii Tubular ReactorDokument33 SeitenCet Mkii Tubular ReactorAdila AnbreenNoch keine Bewertungen
- IYOHA COLLINS 16CF020531 Batch Reactor ReportDokument19 SeitenIYOHA COLLINS 16CF020531 Batch Reactor ReportDavid OvieNoch keine Bewertungen
- CSTR FinalDokument36 SeitenCSTR FinalMuhammad Yar KhanNoch keine Bewertungen
- Practical Chemical Thermodynamics for GeoscientistsVon EverandPractical Chemical Thermodynamics for GeoscientistsNoch keine Bewertungen
- Physical and Chemical Equilibrium for Chemical EngineersVon EverandPhysical and Chemical Equilibrium for Chemical EngineersBewertung: 5 von 5 Sternen5/5 (1)
- Handbook of Thermal Conductivity, Volume 1: Organic Compounds C1 to C4Von EverandHandbook of Thermal Conductivity, Volume 1: Organic Compounds C1 to C4Bewertung: 5 von 5 Sternen5/5 (1)
- Gas Absorption Principles and Column Design ModelsDokument20 SeitenGas Absorption Principles and Column Design Modelsgzairene8762100% (1)
- JKHJKHJKHJK HDokument1 SeiteJKHJKHJKHJK Hgzairene8762Noch keine Bewertungen
- Sulfuric AcidDokument4 SeitenSulfuric Acidgzairene8762Noch keine Bewertungen
- Specification DatacommDokument1 SeiteSpecification Datacommgzairene8762Noch keine Bewertungen
- Rile HandbookDokument3 SeitenRile Handbookgzairene8762Noch keine Bewertungen
- Http://en - wikibooks.org/Wiki/Control Systems/Transfer Functions HTTP://WWW - ehow.Com/About 5117391 Turmeric-Extract - HTMLDokument1 SeiteHttp://en - wikibooks.org/Wiki/Control Systems/Transfer Functions HTTP://WWW - ehow.Com/About 5117391 Turmeric-Extract - HTMLgzairene8762Noch keine Bewertungen
- Http://en - wikibooks.org/Wiki/Control Systems/Transfer Functions HTTP://WWW - ehow.Com/About 5117391 Turmeric-Extract - HTMLDokument1 SeiteHttp://en - wikibooks.org/Wiki/Control Systems/Transfer Functions HTTP://WWW - ehow.Com/About 5117391 Turmeric-Extract - HTMLgzairene8762Noch keine Bewertungen
- 31.2-General Wave Popeties-Cie Igcse Physics Ext-Theory-QpDokument12 Seiten31.2-General Wave Popeties-Cie Igcse Physics Ext-Theory-Qpnityam bajajNoch keine Bewertungen
- Titration CurveDokument12 SeitenTitration Curveoguztop10Noch keine Bewertungen
- Cables: Compensating and ExtensionDokument32 SeitenCables: Compensating and ExtensionAyadi_AymanNoch keine Bewertungen
- Fire Resistant Fluid Study on Deterioration of Physicochemical PropertiesDokument34 SeitenFire Resistant Fluid Study on Deterioration of Physicochemical Propertiesgolden430Noch keine Bewertungen
- Viscosity & Viscosity Modifiers: © 2019 Infineum International Limited. All Rights Reserved. 2017160Dokument42 SeitenViscosity & Viscosity Modifiers: © 2019 Infineum International Limited. All Rights Reserved. 2017160Jahmia Coralie100% (1)
- US Crete HF - 2020Dokument2 SeitenUS Crete HF - 2020kemdoNoch keine Bewertungen
- 05 S and P Block Elements Que. Final E 1Dokument15 Seiten05 S and P Block Elements Que. Final E 1gnkstarNoch keine Bewertungen
- Plain and Reinforced Concrete (CE-310) : Specific Gravity & Water Absorption of Coarse AggregateDokument14 SeitenPlain and Reinforced Concrete (CE-310) : Specific Gravity & Water Absorption of Coarse AggregateAbdul BasitNoch keine Bewertungen
- Heat Exchanger Design Lecture - 07Dokument24 SeitenHeat Exchanger Design Lecture - 07Mohmmad ShaikhNoch keine Bewertungen
- HEAT TRANSFER LAB REPORTDokument45 SeitenHEAT TRANSFER LAB REPORTnighatNoch keine Bewertungen
- Article 4 (Recovered)Dokument6 SeitenArticle 4 (Recovered)adriannatsNoch keine Bewertungen
- Electric Field Strength and PotentialDokument4 SeitenElectric Field Strength and PotentialAsma Akter100% (1)
- Work Power Energy JEE TestDokument6 SeitenWork Power Energy JEE TestAman RolandNoch keine Bewertungen
- Unit 10 Elements of Group: OccurrenceDokument22 SeitenUnit 10 Elements of Group: OccurrenceSahil JaglanNoch keine Bewertungen
- Earth Materials and ProcessesDokument60 SeitenEarth Materials and ProcessesZarlene SierraNoch keine Bewertungen
- P131 Problem Set 1Dokument2 SeitenP131 Problem Set 1TyNoch keine Bewertungen
- AIChE Pocket HandbookDokument64 SeitenAIChE Pocket HandbookDinesh KanaujiyaNoch keine Bewertungen
- Performance of CI Engines Using Biodiesel As Fuel: January 2009Dokument14 SeitenPerformance of CI Engines Using Biodiesel As Fuel: January 2009Diyar NezarNoch keine Bewertungen
- NSS Chemistry Part 15 Analytical Chemistry (Structural QuestionsDokument42 SeitenNSS Chemistry Part 15 Analytical Chemistry (Structural QuestionsKelvinNgNoch keine Bewertungen
- 2017 - Product Specification - RZBC (JUXIAN) - CAADokument1 Seite2017 - Product Specification - RZBC (JUXIAN) - CAAediasianagri100% (1)
- Pascoe 2005Dokument7 SeitenPascoe 2005mohamadhosein mohamadiNoch keine Bewertungen
- Safari - 6 Sep 2022 at 2:40 PMDokument1 SeiteSafari - 6 Sep 2022 at 2:40 PMTerrence AzariaNoch keine Bewertungen
- 08.solutions To ConceptsDokument16 Seiten08.solutions To ConceptsPallav Jyoti PalNoch keine Bewertungen
- Astm D971Dokument4 SeitenAstm D971JORGE SANTANDERNoch keine Bewertungen
- Problem Set 1: Dynamic Physics Problems/TITLEDokument3 SeitenProblem Set 1: Dynamic Physics Problems/TITLEpeneNoch keine Bewertungen
- NMR for sequence and structural isomerism analysisDokument19 SeitenNMR for sequence and structural isomerism analysisSyed Hashim Shah HashmiNoch keine Bewertungen
- Heat Transfer PDFDokument3 SeitenHeat Transfer PDFTahmeed AzizNoch keine Bewertungen
- 3 - Gravimetric Analysis of Calcium and Hard Water - S PDFDokument6 Seiten3 - Gravimetric Analysis of Calcium and Hard Water - S PDFJon CranNoch keine Bewertungen
- Globle WarmingDokument87 SeitenGloble WarmingVijay Maurya100% (1)
- Set 1Dokument18 SeitenSet 1RON MARK EDWARD ANDALUZNoch keine Bewertungen