Beruflich Dokumente
Kultur Dokumente
10 Askeland Chap
Hochgeladen von
Ingrid Zetina TejeroOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
10 Askeland Chap
Hochgeladen von
Ingrid Zetina TejeroCopyright:
Verfügbare Formate
10
Dispersion Strengthening and Eutectic Phase Diagrams
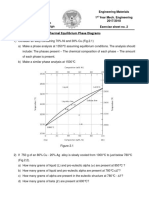
1022 A hypothetical phase diagram is shown in Figure 1032. (a) Are any intermetallic compounds present? If so, identify them and determine whether they are stoichiometric or nonstoichiometric. (b) Identify the solid solutions present in the system. Is either material A or B allotropic? Explain. (c) Identify the three-phase reactions by writing down the temperature, the reaction in equation form, the composition of each phase in the reaction, and the name of the reaction. Solution: (a) u non-stoichiometric intermetallic compound.
(b) a, h, g, and b; material B is allotropic, existing in three different forms at different temperatures (c) 1100C: 900C: 680C: 600C: g L S b; peritectic; L: 82% B g: 97% B b: 90% B
L1 S L2 LSa a
a; monotectic; L1: 28% B L 2: 50% B a: 5% B b; eutectic; peritectoid; eutectoid; L: 60% B a: 5% B b: 90% B a: 5% B b: 80% B u: 37% B b: 90% B u: 40% B h: 95% B
b S u; h;
300C: b S u
1023 The CuZn phase diagram is shown in Figure 1033. (a) Are any intermetallic compounds present? If so, identify them and determine whether they are stoichiometric or nonstoichiometric. (b) Identify the solid solutions present in the system. (c) Identify the three-phase reactions by writing down the temperature, the reaction in equation form, and the name of the reaction.
111
112
The Science and Engineering of Materials
Instructors Solution Manual
Solution: (a) b, b , g, d, e: all nonstoichiometric. (b) a, u (c) 900C: a L S b; L S g; L S d; L S e; e; peritectic peritectic peritectic peritectic eutectoid peritectic
830C: b 700C: 600C: 550C: 420C: g d
d S g e
L S u;
250C: b S a
g; eutectoid
1024 A portion of the AlCu phase diagram is shown in Figure 1034. (a) Determine the formula for the u compound. (b) Identify the three-phase reaction by writing down the temperature, the reaction in equation form, the composition of each phase in the reaction, and the name of the reaction. 54 g 63.54 g/mol 54 63.54 46 26.981
Solution: (a) u at 54% Cu; (b) 548C; L S a
33 at% Cu; CuAl2
u; eutectic; L: 33.2% Cu, a: 5.65% Cu, u: 52.5% Cu.
1025 The AlLi phase diagram is shown in Figure 1035. (a) Are any intermetallic compounds present? If so, identify them and determine whether they are stoichiometric or nonstoichiometric. Determine the formula for each compound. (b) Identify the three-phase reactions by writing down the temperature, the reaction in equation form, the composition of each phase in the reaction, and the name of the reaction. Solution: (a) b is non-stoichiometric @ 21 wt% Li: at% Li 21 g 6.94 g/mol 21 6.94 79 26.981 100% 50 at% Li AlLi
g, is stoichiometric @ 34 wt% Li: at% Li 34 g 6.94 g/mol 34 6.94 66 26.981 b eutectic peritectic eutectic 100% L: 9.9% Li a: 4% Li b: 20.4% Li 510C: b L S g b: 25% Li L: 47% Li g: 34% Li 170C: L S g a 1Li2 L: 98% Li g: 34% Li a 1Li2: 99% Li 66.7% Li AlLi2
(b) 600C: L S a
1026 An intermetallic compound is found for 10 wt% Si in the CuSi phase diagram. Determine the formula for the compound. 10 g 28.08 g/mol 10 28.08 90 63.54
Solution:
at% Si
0.20 or SiCu4
CHAPTER 10
Dispersion Strengthening and Eutectic Phase Diagrams
113
1027 Using the phase rule, predict and explain how many solid phases will form in an eutectic reaction in a ternary (three-component) phase diagram, assuming that the pressure is fixed. Solution: F C P 1 0, C b 3 0 3 P 1 or P 4
At the eutectic, F Therefore, L S a
g and 3 solid phases form.
1030 Consider a Pb15% Sn alloy. During solidification, determine (a) the composition of the first solid to form, (b) the liquidus temperature, solidus temperature, solvus temperature, and freezing range of the alloy, (c) the amounts and compositions of each phase at 260C, (d) the amounts and compositions of each phase at 183C, and (e) the amounts and compositions of each phase at 25C. Solution: (a) 8% Sn (b) liquidus solvus 290C, solidus 240C, 170C, freezing range 50C
(c) L: 30% Sn a: 12% Sn; 15 12 %L 100% 30 12 (d) a: 15% Sn 100% a (e) a: 2% Pb b: 100% Sn 100 15 %a 100 100 2
17%
%a
83%
87%
%b
13%
1031 Consider an Al12% Mg alloy (Figure 1036). During solidification, determine (a) the composition of the first solid to form, (b) the liquidus temperature, solidus temperature, solvus temperature, and freezing range of the alloy, (c) the amounts and compositions of each phase at 525C, (d) the amounts and compositions of each phase at 450C, and (e) the amounts and compositions of each phase at 25C. Solution: (a) 2.5% Mg (b) liquidus solvus 600C, solidus 470C, 400C, freezing range 130C
(c) L: 26% Mg a: 7% Mg; 26 12 %a 100% 26 7 (d) a: 12% Mg 100% a (e) a: 1% Mg b: 34% Mg 34 12 100% %a 34 1
74%
%L
26%
67%
%b
33%
114
The Science and Engineering of Materials
Instructors Solution Manual
1032 Consider a Pb35% Sn alloy. Determine (a) if the alloy is hypoeutectic or hypereutectic, (b) the composition of the first solid to form during solidification, (c) the amounts and compositions of each phase at 184C, (d) the amounts and compositions of each phase at 182C, (e) the amounts and compositions of each microconstituent at 182C, and (f) the amounts and compositions of each phase at 25C. Solution: (a) hypoeutectic (b) 14% Sn
(c) a: 19% Sn L: 61.9% Sn 61.9 35 %a 100% 61.9 19 (d) a: 19% Sn b: 97.5% Sn 97.5 35 %a 100% 97.5 19
63%
%L
37%
80%
%b 63% 37%
20%
(e) primary a: 19% Sn %primary a eutectic: 61.9% Sn %eutectic (f) a: 2% Sn b: 100% Sn 100 35 %a 100% 100 2
66%
%b
34%
1033 Consider a Pb70% Sn alloy. Determine (a) if the alloy is hypoeutectic or hypereutectic, (b) the composition of the first solid to form during solidification, (c) the amounts and compositions of each phase at 184C, (d) the amounts and compositions of each phase at 182C, (e) the amounts and compositions of each microconstituent at 182C, and (f) the amounts and compositions of each phase at 25C. Solution: (a) hypereutectic (b) 98% Sn
L: 61.9% Sn (c) b: 97.5% Sn 70 61.9 %b 100% 97.5 61.9 (d) a: 19% Sn 97.5 %a 97.5 b: 97.5% Sn 70 100% 19
22.8%
%L
77.2%
35%
%b 22.8% 77.2%
65%
(e) primary b: 97.5% Sn %primary b eutectic: 61.9% Sn %eutectic (f) a: 2% Sn b: 100% Sn 100 70 100% %a 100 2
30%
%b
70%
1034 Calculate the total % b and the % eutectic microconstituent at room temperature for the following lead-tin alloys: 10% Sn, 20% Sn, 50% Sn, 60% Sn, 80% Sn, and 95% Sn. Using Figure 1022, plot the strength of the alloys versus the % b and the % eutectic and explain your graphs.
CHAPTER 10 Solution:
Dispersion Strengthening and Eutectic Phase Diagrams %b 10% Sn 20% Sn 50% Sn 60% Sn 80% Sn 95% Sn 10 99 20 99 50 99 60 99 80 99 95 99 2 2 2 2 2 2 2 2 2 2 2 2 8.2% 18.6% 49.5% 59.8% 80.4% 95.9% 20 19 61.9 19 50 19 61.9 19 60 19 61.9 19 97.5 80 97.5 61.9 97.5 95 97.5 61.9 %eutectic 0% 2.3% 72.3% 95.6% 49.2% 7.0%
115
8000
8000
Tensile strength (psi)
Tensile strength (psi)
7000
7000
oHyp
6000
Hy pe r-
6000
5000
5000
4000 20 40 % 60 80 100 20 40 60 % eutectic 80
1035 Consider an Al4% Si alloy. (See Figure 1023.) Determine (a) if the alloy is hypoeutectic or hypereutectic, (b) the composition of the first solid to form during solidification, (c) the amounts and compositions of each phase at 578C, (d) the amounts and compositions of each phase at 576C, the amounts and compositions of each microconstituent at 576C, and (e) the amounts and compositions of each phase at 25C. Solution: (a) hypoeutectic (b) 1% Si (c) a: 1.65% Si L: 12.6% Si 12.6 4 %a 78.5% 12.6 1.65 (d) a: 1.65% Si b: 99.83% Si 99.83 4 97.6% %a 99.83 1.65
%L
21.5%
%b
2.4%
116
The Science and Engineering of Materials
Instructors Solution Manual %primary a %eutectic %a 100 100 78.5% 21.5% 4 0 96% %b 4%
(e) primary a: 1.65% Si eutectic: 12.6% Si a: 0% Si b: 100% Si
1036 Consider a Al25% Si alloy. (See Figure 1023.) Determine (a) if the alloy is hypoeutectic or hypereutectic, (b) the composition of the first solid to form during solidification, (c) the amounts and compositions of each phase at 578C, (d) the amounts and compositions of each phase at 576C, (e) the amounts and compositions of each microconstituent at 576C, and (f) the amounts and compositions of each phase at 25C. Solution: (a) hypereutectic (b) 100% Si (c) b: 99.83% Si L: 12.6% Si 99.83 25 %L 85.8% 99.83 12.6 (d) a: 1.65% Si b: 99.83% Si 99.83 25 %a 76.2% 99.83 1.65
%b
14.2%
%b
23.8% 14.2% 85.8% 25 0 75% %b 25%
(e) primary b: 99.83% Si %primary b eutectic: 12.6% Si %eutectic (f) a: 0% Si b: 100% Si %a 100 100
1037 A PbSn alloy contains 45% a and 55% b at 100C. Determine the composition of the alloy. Is the alloy hypoeutectic or hypereutectic? Solution: 98.0 98.0 x 5 100 or x 56.15% Sn Hypoeutectic
%a
45
1038 An AlSi alloy contains 85% a and 15% b at 500C. Determine the composition of the alloy. Is the alloy hypoeutectic or hypereutectic? Solution: %a 85 100 100 x 1 100
or x
15.85% Si Hypereutectic
1039 A PbSn alloy contains 23% primary a and 77% eutectic microconstituent. Determine the composition of the alloy. Solution: %primary a 61.9 61.9 x 19 100 or x 52% Sn
23
1040 An AlSi alloy contains 15% primary b and 85% eutectic microconstituent. Determine the composition of the alloy.
CHAPTER 10
Dispersion Strengthening and Eutectic Phase Diagrams 100 x 100 12.6
117
Solution:
%eutectic
85
100
or x
25.71% Si
1041 Determine the maximum solubility for the following cases. (a) (b) (c) (d) lithium in aluminum (Figure 1035), aluminum in magnesium (Figure 1037), copper in zinc (Figure 1033), and carbon in g-iron (Figure 1038)
Solution: (a) 4% Li dissolves in aluminum (b) 12.7% Al dissolves in magnesium (c) 3% Cu dissolves in zinc (d) 2.11% C dissolves in g-iron 1042 Determine the maximum solubility for the following cases. (a) (b) (c) (d) magnesium in aluminum (Figure 1036), zinc in copper (Figure 1033), beryllium in copper (Figure 1033), and Al2O3 in MgO (Figure 1039)
Solution: (a) 14.9% Mg dissolves in aluminum (b) 40% Zn dissolves in copper (c) 2.5% Be dissolves in copper (d) 18% Al2O3 dissolves in MgO 1043 Observation of a microstructure shows that there is 28% eutectic and 72% primary b in an AlLi alloy (Figure 1035). (a) Determine the composition of the alloy and whether it is hypoeutectic or hypereutectic. (b) How much a and b are in the eutectic microconstituent? Solution: (a) 28 20.4 20.4 x 9.9 9.9 4 100 or x 17.46% Li Hypereutectic
(b) %aEut
20.4 20.4
100%
64%
and
%bEut
36%
1044 Write the eutectic reaction that occurs, including the compositions of the three phases in equilibrium, and calculate the amount of a and b in the eutectic microconstituent in the MgAl system, (Figure 1036). Solution: L32.3 S a12.7 %aEut g40.2 40.2 40.2 32.3 12.7 100% 28.7% and %gEut 71.3%
118
The Science and Engineering of Materials
Instructors Solution Manual
1045 Calculate the total amount of a and b and the amount of each microconstituent in a Pb50% Sn alloy at 182C. What fraction of the total a in the alloy is contained in the eutectic microconstituent? Solution: atotal aPrimary ain eutectic f 97.5 97.5 61.9 61.9 60.5 50 19 50 19 27.7 100% 100% 32.8% 0.54 60.5% 27.7% bTotal Eutectic 39.5% 72.3%
32.8 60.5
1046 Figure 1040 shows a cooling curve for a PbSn alloy. Determine (a) the pouring temperature, (b) the superheat, (c) the liquidus temperature, (d) the eutectic temperature, (e) the freezing range, (f) the local solidification time, (g) the total solidification time, and (h) the composition of the alloy. Solution: (a) pouring temperature (b) superheat 360 360C 250 110C
(c) liquidus temperature (d) eutectic temperature (e) freezing range 250
250C 183C 183 600 600 s 67C 110 490 s
(f) local solidification time (g) total solidification time (h) approximately 32% Sn
1047 Figure 1041 shows a cooling curve for an AlSi alloy. Determine (a) the pouring temperature, (b) the superheat, (c) the liquidus temperature, (d) the eutectic temperature, (e) the freezing range, (f) the local solidification time, (g) the total solidification time, and (h) the composition of the alloy. Solution: (a) pouring temperature (b) superheat 1150 1150C 1000 150C
(c) liquidus temperature (d) eutectic temperature (e) freezing range 1000
1000C 577C 577 11.5 423C 1 10.5 min
(f) local solidification time (g) total solidification time (h) approximately 45% Si
11.5 min
CHAPTER 10
Dispersion Strengthening and Eutectic Phase Diagrams
119
1048 Draw the cooling curves, including appropriate temperatures, expected for the following AlSi alloys. (a) Al4% Si Solution: (b) Al12.6% Si (c) Al25% Si (d) Al65% Si
630
780 577 577
1200 577
T
Al 12.6% Si t
577 Al 65% Si t
Al 4% Si t
Al 25% Si t
1049 Based on the following observations, construct a phase diagram. Element A melts at 850C and element B melts at 1200C. Element B has a maximum solubility of 5% in element A, and element A has a maximum solubility of 15% in element B. The number of degrees of freedom from the phase rule is zero when the temperature is 725C and there is 35% B present. At room temperature 1% B is soluble in A and 7% A is soluble in B. Solution:
1200 1000 Temperature (C) 800 a 600 400 200 a+b a+L
L
b+L b
A 5
20
40 %B
60
80 85
120
The Science and Engineering of Materials
Instructors Solution Manual
1050 Cooling curves are obtained for a series of CuAg alloys, (Figure 1042). Use this data to produce the CuAg phase diagram. The maximum solubility of Ag in Cu is 7.9% and the maximum solubility of Cu in Ag is 8.8%. The solubilities at room temperature are near zero. Solution: 0% Ag 8% Ag 20% Ag 50% Ag 71.9% Ag 90% Ag 100% Ag Tliq S 1085C S 1030C S 975C S 860C S 780C S 870C S 961C Tsol 950C 780C 780C 780C 780C
1100
Temperature (C)
1000
900 a 800 a+b 700 Cu 29 40 60 a+L
L
b+L b
80
Ag
%Ag
1051 The SiO2 Al2O3 phase diagram is included in Figure 1027(b). A refractory is required to contain molten metal at 1900C. (a) Will pure Al2O3 be a potential candidate? Explain. (b) Will Al2O3 contaminated with 1% SiO2 be a candidate? Explain. Solution: (a) Yes. Tm 2040C 1900C No liquid will form.
(b) No. Some liquid will form. %L 100 100 99 80 100% 5% L
This liquid will weaken the refractory.
CHAPTER 10
Dispersion Strengthening and Eutectic Phase Diagrams
121
1066 Consider the ternary phase diagram shown in Figures 1030 and 1031. Determine the liquidus temperature, the first solid to form, and the phases present at room temperature for the following compositions. (a) 30% B20% C, balance A (c) 60% B10% C, balance A Solution: (a) TLiq (b) TLiq (c) TLiq (b) 10% B25% C, balance A
220C; b; a 330C; a; a 390C; b; a
g g b
1067 Consider the ternary phase diagram shown in Figures 1030 and 1031. Determine the liquidus temperature, the first solid to form, and the phases present at room temperature for the following compositions. (a) 5% B80% C, balance A (c) 30% B35% C, balance A Solution: (a) TLiq (b) TLiq (c) TLiq (b) 50% B5% C, balance A
390C; g; a 330C; b; a 290C; b; a
g b b g
1068 Consider the liquidus plot in Figure 1030. (a) For a constant 20% B, draw a graph showing how the liquidus temperature changes from 20% B0% C, balance A to 20% B80% C, balance A, (b) What is the composition of the ternary eutectic in this system? (c) Estimate the temperature at which the ternary eutectic reaction occurs. Solution: %A %B %C 80200 702010 602020 502030 402040 302050 202060 102070 02080 Tliquidus 390 C 355C 300C 210C 150C 210C 270C 320C 400C
400 Temperature (C)
L
300 200 100 a+L g+L
B = 20%
0 20 40 %C 60 80
122
The Science and Engineering of Materials
Instructors Solution Manual
(b) The composition of the ternary eutectic is about 40% 20% B40% C, balance A (c) The ternary eutectic temperature is about 150C 1069 From the liquidus plot in Figure 1030, prepare a graph of liquidus temperature versus percent B for a constant ratio of materials A and C (that is, from pure B to 50% A50% C on the liquidus plot). Material B melts at 600C. Solution: A B C 50 050 451045 402040 353035 304030 255025 206020 157015 01000 200C 180C 150C 280C 330C 375C 415C 485C 580C
600 500 Temperature (C) 400 300 200 a+L 100 %A=%C
L
b+L
20
40 %B
60
80
100
Das könnte Ihnen auch gefallen
- Askeland Science and Engineering 7e ISM Chapter 10Dokument26 SeitenAskeland Science and Engineering 7e ISM Chapter 10Ian Gabriel Cañas Fernández100% (1)
- Askeland Science and Engineering 7e ISM Chapter 08Dokument24 SeitenAskeland Science and Engineering 7e ISM Chapter 08Ian Gabriel Cañas Fernández100% (4)
- Askeland Science and Engineering 7e ISM Chapter 09Dokument28 SeitenAskeland Science and Engineering 7e ISM Chapter 09Ian Gabriel Cañas Fernández100% (1)
- 05 Askeland ChapDokument10 Seiten05 Askeland ChapWeihanZhang100% (1)
- Atomic and Ionic Arrangements CalculationsDokument19 SeitenAtomic and Ionic Arrangements CalculationsRafael AraújoNoch keine Bewertungen
- 08 Askeland Chap PDFDokument12 Seiten08 Askeland Chap PDFMaiefnbNoch keine Bewertungen
- 20 Askeland ChapDokument10 Seiten20 Askeland Chapel_dualcNoch keine Bewertungen
- Construction Materials Density and Dimensional ChangesDokument4 SeitenConstruction Materials Density and Dimensional ChangesIngrid Zetina Tejero100% (1)
- Problems Phase FeDokument9 SeitenProblems Phase FeAshutoshKumarNoch keine Bewertungen
- 21 Askeland ChapDokument8 Seiten21 Askeland Chapel_dualcNoch keine Bewertungen
- Defect ProblemsDokument8 SeitenDefect Problemsndreddy_pu100% (2)
- Solidification and Crystalline ImperfectionsDokument43 SeitenSolidification and Crystalline ImperfectionsArnaldo Bester67% (3)
- Askeland Science and Engineering 7e ISM Chapter 05Dokument24 SeitenAskeland Science and Engineering 7e ISM Chapter 05Ian Gabriel Cañas Fernández50% (2)
- 11 Askeland ChapDokument12 Seiten11 Askeland Chapsergalan100% (1)
- Assignment 7 SolutionsDokument11 SeitenAssignment 7 SolutionsIsaiah Qe LiewNoch keine Bewertungen
- Mech 285 True or False Quiz ReviewDokument18 SeitenMech 285 True or False Quiz ReviewKayleigh RobotnikNoch keine Bewertungen
- Materials Engineering QuestionsDokument2 SeitenMaterials Engineering QuestionsNicole Mae AllosadaNoch keine Bewertungen
- Chapt 03 Sect 7 To 11Dokument15 SeitenChapt 03 Sect 7 To 11Jesse McClure100% (1)
- Chapter 14: Nonferrous AlloysDokument12 SeitenChapter 14: Nonferrous AlloysIan Gabriel Cañas FernándezNoch keine Bewertungen
- Chapter 7 HW SolutionsDokument11 SeitenChapter 7 HW SolutionslesleyNoch keine Bewertungen
- SOLUTIONS MANUAL FOR ENGINEERING MATERIALS IDokument43 SeitenSOLUTIONS MANUAL FOR ENGINEERING MATERIALS ISadiq Omar100% (1)
- Askeland Science and Engineering 7e ISM Chapter 03Dokument52 SeitenAskeland Science and Engineering 7e ISM Chapter 03Michael Ernesto Arias Mendez100% (4)
- Chapt 11Dokument30 SeitenChapt 11Ben NweeangNoch keine Bewertungen
- Chapt 07Dokument54 SeitenChapt 07Jesse McClure80% (10)
- 19 Askeland ChapDokument6 Seiten19 Askeland Chapel_dualcNoch keine Bewertungen
- Exercícios CallisterDokument66 SeitenExercícios CallistermicrovilosidadesNoch keine Bewertungen
- Phase Diagrams: Solubility LimitDokument163 SeitenPhase Diagrams: Solubility LimitseaNoch keine Bewertungen
- HW8 Soln PDFDokument9 SeitenHW8 Soln PDFPatricia de Leon100% (1)
- Assign 2 SolutionsDokument7 SeitenAssign 2 SolutionsAnshu Kumar Gupta100% (3)
- 3a - 8.1 A 8.11FRATURA E FADIGA PDFDokument70 Seiten3a - 8.1 A 8.11FRATURA E FADIGA PDFmatheus oliveira100% (6)
- Chapt 04Dokument22 SeitenChapt 04Jesse McClureNoch keine Bewertungen
- Tablas de ConversionDokument5 SeitenTablas de ConversionFisicoquimica ULSNoch keine Bewertungen
- HW 9 2011 SolutionsDokument15 SeitenHW 9 2011 SolutionsParamesh Anugu100% (1)
- Assign 3 SolutionsDokument5 SeitenAssign 3 SolutionsAnshu Kumar Gupta100% (3)
- Closed-Book Practice-Ch 09 (2017!08!07)Dokument9 SeitenClosed-Book Practice-Ch 09 (2017!08!07)Juan100% (1)
- Chapter 3: Atomic and Ionic Arrangements: 3-1 SolutionDokument52 SeitenChapter 3: Atomic and Ionic Arrangements: 3-1 SolutionMarcos Jose100% (1)
- Capitulo III Combustion ButssDokument40 SeitenCapitulo III Combustion ButssAnonymous uKnpMkRNoch keine Bewertungen
- Chapt 08Dokument21 SeitenChapt 08Jesse McClure100% (5)
- Chapter 8 - Phase Diagram PART2Dokument26 SeitenChapter 8 - Phase Diagram PART2Mohd IqbalNoch keine Bewertungen
- Assignment PhaseDiaDokument5 SeitenAssignment PhaseDiaAnshu Kumar GuptaNoch keine Bewertungen
- Ch-27.3 Iron Carbon Equilibrium DiagramDokument20 SeitenCh-27.3 Iron Carbon Equilibrium DiagramasjfgauojfgfNoch keine Bewertungen
- Che 3330 - Spring 2012 HW 5Dokument5 SeitenChe 3330 - Spring 2012 HW 5Brett CasserlyNoch keine Bewertungen
- Engineering Materials Phase DiagramsDokument6 SeitenEngineering Materials Phase DiagramsOmar AssalNoch keine Bewertungen
- MLE1101 Tutorial 4 - Suggested Solutions AnalysisDokument7 SeitenMLE1101 Tutorial 4 - Suggested Solutions AnalysisYin HauNoch keine Bewertungen
- Binary Alloy Phase DiagramsDokument12 SeitenBinary Alloy Phase Diagramseeng.ali651550% (2)
- Soal Diagram FasaDokument1 SeiteSoal Diagram FasaDecy Putri EfhanaNoch keine Bewertungen
- Phase Diagram Exercises - Worked Answers - CorrectedDokument14 SeitenPhase Diagram Exercises - Worked Answers - CorrectedHayden Reeves75% (4)
- Assignments On Phase Equilibrium in CeramicsDokument5 SeitenAssignments On Phase Equilibrium in CeramicsDESALEGN SHIBESHNoch keine Bewertungen
- Chem PPRDokument4 SeitenChem PPRJitendra KaushikNoch keine Bewertungen
- Week 11Dokument12 SeitenWeek 11lduran_63Noch keine Bewertungen
- 98materials Phase DiagramsDokument27 Seiten98materials Phase DiagramsHari PrasathNoch keine Bewertungen
- Exam 1 PracDokument5 SeitenExam 1 PracAron StubbsNoch keine Bewertungen
- Uace Chemistry Seminar UpdatedDokument21 SeitenUace Chemistry Seminar UpdatedAnonymous 0aXUKMTqV100% (1)
- GTU BE Semester III Process Calculation Exam QuestionsDokument2 SeitenGTU BE Semester III Process Calculation Exam QuestionskhushbuNoch keine Bewertungen
- Tutorial 2 HelperDokument3 SeitenTutorial 2 HelperDedy SaputraNoch keine Bewertungen
- Tutorial Problems 5Dokument8 SeitenTutorial Problems 5Majak MarialNoch keine Bewertungen
- General Chemistry I Chapter 1 -16 Practice Questions SolvedDokument6 SeitenGeneral Chemistry I Chapter 1 -16 Practice Questions SolvedHajime Hikari100% (1)
- Quiz 5 SolutionsDokument2 SeitenQuiz 5 SolutionsAkshay SapraNoch keine Bewertungen
- Sample/practice Exam 2010, Questions and Answers Sample/practice Exam 2010, Questions and AnswersDokument14 SeitenSample/practice Exam 2010, Questions and Answers Sample/practice Exam 2010, Questions and AnswersEilyza Aballa100% (1)
- Construction Materials Density and Dimensional ChangesDokument4 SeitenConstruction Materials Density and Dimensional ChangesIngrid Zetina Tejero100% (1)
- 14 Askeland ChapDokument4 Seiten14 Askeland ChapIngrid Zetina TejeroNoch keine Bewertungen
- 11 Askeland ChapDokument12 Seiten11 Askeland Chapsergalan100% (1)
- Strain Hardening and Metal DeformationDokument12 SeitenStrain Hardening and Metal DeformationessahhNoch keine Bewertungen
- CH 07Dokument40 SeitenCH 07Ambreen31Noch keine Bewertungen
- Table of Contents: MechatronicsDokument45 SeitenTable of Contents: MechatronicsFabo Barajas100% (1)
- PandoraFMS Alerts WhatsappDokument6 SeitenPandoraFMS Alerts Whatsapppacolo89Noch keine Bewertungen
- Mechanical Vapor RecompressionDokument9 SeitenMechanical Vapor Recompressionnarayana reddy0% (1)
- Public Dealing With UrduDokument5 SeitenPublic Dealing With UrduTariq Ghayyur86% (7)
- Systems Analyst Interview Questions GuideDokument3 SeitenSystems Analyst Interview Questions GuidehassanshoaibNoch keine Bewertungen
- High Current Transistor SpecsDokument5 SeitenHigh Current Transistor SpecsamernasserNoch keine Bewertungen
- Ata 47-NGS R25Dokument148 SeitenAta 47-NGS R25NadirNoch keine Bewertungen
- Mohit Chakrabarti - Value Education - Changing PerspectivesDokument79 SeitenMohit Chakrabarti - Value Education - Changing PerspectivesDsakthyvikky100% (1)
- Mastering EES Chapter1Dokument66 SeitenMastering EES Chapter1mianvaherNoch keine Bewertungen
- Work Instruction For Coil Taping Process of EE14 (1&4 Pin)Dokument6 SeitenWork Instruction For Coil Taping Process of EE14 (1&4 Pin)k.mehaboob bashaNoch keine Bewertungen
- Ticket SunilDokument2 SeitenTicket SunilDURGA PRASAD TRIPATHYNoch keine Bewertungen
- Concept MappingDokument8 SeitenConcept MappingRashid LatiefNoch keine Bewertungen
- A2 Slot CAT2 QPDokument1 SeiteA2 Slot CAT2 QPSeverus SnapeNoch keine Bewertungen
- Manual de Diagnostico D3E PDFDokument72 SeitenManual de Diagnostico D3E PDFJosé Luis Contreras Calderón100% (3)
- Cascadable Broadband Gaas Mmic Amplifier DC To 10Ghz: FeaturesDokument9 SeitenCascadable Broadband Gaas Mmic Amplifier DC To 10Ghz: Featuresfarlocco23Noch keine Bewertungen
- Timecode and SourcesDokument4 SeitenTimecode and Sourcesapi-483055750Noch keine Bewertungen
- Aadhaar is proof of identity, not citizenshipDokument1 SeiteAadhaar is proof of identity, not citizenshipPARTAPNoch keine Bewertungen
- Unit 30 WorkDokument2 SeitenUnit 30 WorkThanh HàNoch keine Bewertungen
- Mahusay Module 4 Acc3110Dokument2 SeitenMahusay Module 4 Acc3110Jeth MahusayNoch keine Bewertungen
- DX DiagesDokument36 SeitenDX DiagesBpbd Kota BengkuluNoch keine Bewertungen
- ACI-439.3R-91 Mechanical Connections of Reinforcing BarsDokument16 SeitenACI-439.3R-91 Mechanical Connections of Reinforcing BarsMichi AGNoch keine Bewertungen
- Ausa 300-RH - en Parts ManualDokument93 SeitenAusa 300-RH - en Parts ManualandrewhNoch keine Bewertungen
- Assignment (40%) : A) Formulate The Problem As LPM B) Solve The LPM Using Simplex AlgorithmDokument5 SeitenAssignment (40%) : A) Formulate The Problem As LPM B) Solve The LPM Using Simplex Algorithmet100% (1)
- Asphalt Laboratory Manual RevDokument13 SeitenAsphalt Laboratory Manual RevKurian C ChackoNoch keine Bewertungen
- COE 107.04 Cathodic Protection Monitoring Instruments and ProceduresDokument42 SeitenCOE 107.04 Cathodic Protection Monitoring Instruments and ProceduresMo'tasem Serdaneh100% (1)
- Artificial MusclesDokument8 SeitenArtificial MusclespinnakapentiumNoch keine Bewertungen
- Nordson EFD Ultimus I II Operating ManualDokument32 SeitenNordson EFD Ultimus I II Operating ManualFernando KrauchukNoch keine Bewertungen
- Yoshimi Advanced User ManualDokument297 SeitenYoshimi Advanced User Manualfby999Noch keine Bewertungen
- WHLP G9 ESolomon Nov 23-27Dokument4 SeitenWHLP G9 ESolomon Nov 23-27Ericha SolomonNoch keine Bewertungen