Beruflich Dokumente
Kultur Dokumente
Hydrogen and Oxygen
Hochgeladen von
Bruce KarpeOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Hydrogen and Oxygen
Hochgeladen von
Bruce KarpeCopyright:
Verfügbare Formate
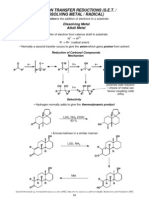
Hydrogen & Oxygen: Generating, Collecting, and Testing
Background Mixtures of hydrogen and oxygen can explode and release considerable energy. This energy may be used to launch a rocket. A contest will be held to see whose rocket can be launched the furthest. Several variables can be tested, including: the ratio of H2 to O2, the amount of water left in the rocket, the angle of launch, etc. Because this activity will be done on a microscale level, the explosions, although potentially loud, are safe; however, the solutions used can cause serious damage should they come in contact with the eyes. SAFETY GOGGLES MUST BE WORN AT ALL TIMES. Materials needed a 250 mL beaker a test tube labeled H2 generator a test tube labeled O2 generator two one-hole rubber stopper with 1-inch nozzles one cut-off plastic pipet candle and matches 3 M hydrochloric acid (HCl) 3 % hydrogen peroxide (H2O2) potassium iodide (KI) solution zinc (3-5 pieces) tap water one permanent marker a wood splint a wax pencil a ruler
Procedure 1. Put your goggles on; they must remain on throughout the lab. 2. Fill the 250 mL beaker about full with tap water. This will act as a test tube holder, a temperature regulator, and a water reserve during the experiment. 3. Take two test tubes, two stoppers with nozzles, a wood splint, and a cut-off pipet from the front desk. 4. Using a wax pencil, label one test tube H2 generator and the other O2 generator. 5. Using a ruler and the permanent marker, mark increments on the cut-off pipet to show six equal-volume intervals.
6. Add the zinc pieces to the H2 generator test tube. 7. Add enough 3 M hydrochloric acid (HCl) to fill the H2 generator to within 2 cm of the top. Place a stopper on top of the test tube, and set the generator in the beaker of water. Wait 5 seconds before beginning the next step. 8. Light a candle. 9. Fill the cutoff pipet completely full with water, and then place the end of the pipet over the nozzle of the generator to collect the hydrogen gas. 10. Once the pipet bulb has become filled with gas, hold it horizontally with its mouth roughly 1 centimeter from the middle of the flame. AVOID PUTTING THE PIPET DIRECTLY IN THE FLAME; IT WILL MELT AND COULD POSSIBLY BURN. Gently squeeze a very small portion of the contents of the pipet into the flame and observe. Repeat. 1 cm
11. Fill the second test tube (the O2 generator) with hydrogen peroxide (H2O2) to within 2 cm of the top. 12. Add 6 10 drops of the potassium iodide solution. Place a stopper on top of the test tube, and set the generator in the beaker of water. Wait 5 seconds before beginning the next step. 13. Fill the cutoff pipet completely full with water, and then place the end of the pipet over the nozzle of the generator to collect the oxygen gas. 14. Once the pipet bulb has become filled with O2 gas, light a wooden splint, and allow it to burn for awhile; then, blow it out so that it is only glowing. 15. Squeeze the bulb over the glowing splint to test for oxygen gas. Repeat. 16. With both gases being generated side by side, collect and test different ratios of hydrogen and oxygen. Be as consistent as possible. Try to find the correct ratio of hydrogen to oxygen. Note: if either of the two reactions slows down too much, simply remove the stopper, carefully decant (pour off) the remaining liquid into the sink, and replace it with some fresh solution from the appropriate containers. Replace the stopper, and wait 5 seconds before resuming the collection of gas. 17. Collect the optimum mixture one more time, but leave a small plug of water in the stem of the pipet. Instead of pop-testing it with the flame, take it to the launch pad. 18. The activation energy for the launch will be supplied by your instructor with a Tesla coil. 19. Think of ways to make your rocket go further, and then try them. 20. Dispose of the zinc as directed. DO NOT POUR THE CONTENTS OF THE H2 DOWN THE DRAIN. You may pour the KI and H2O2 down the sink. Return the equipment, wipe the lab station, and then wash your hands.
Name ___________________________________ Class _______ Date ___________________
Hydrogen & Oxygen: Generating, Collecting, and Testing
Data Tables Pop-test properties of hydrogen gas Pop-test properties of oxygen gas Oxygen:Hydrogen Mole Ratio 1:5 2:4 3:3 4:2 5:1 Post-Lab Questions 1. What is the reaction that takes place side the hydrogen generator?
Relative Loudness
2. What is the reaction that takes place side the oxygen generator?
3. There are two reasons for filling the generators so full. Can you think of them?
4. Did you find any combinations that produced no reaction at all? How could that happen?
5. Write a balanced equation for the reaction between hydrogen and oxygen.
6. What proportion of hydrogen and oxygen produced the most explosive mixture? Why was that mixture most explosive?
7. Why do hydrogen and oxygen in the pipet bulb not react as soon as they mix?
8. What methods did you attempt to make your rocket go further? Which ones worked?
Das könnte Ihnen auch gefallen
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (587)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (73)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (265)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2219)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (119)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- Hinrike Malda - Designing Dendrimers For Use in Biomedical ApplicationsDokument157 SeitenHinrike Malda - Designing Dendrimers For Use in Biomedical ApplicationsHilltopssNoch keine Bewertungen
- Introduction of Environmental GeologyDokument32 SeitenIntroduction of Environmental GeologyAmna SadiqNoch keine Bewertungen
- Chapter 2: Properties of Hydraulic Fluids Example 2.3Dokument13 SeitenChapter 2: Properties of Hydraulic Fluids Example 2.3currjekNoch keine Bewertungen
- Introduction to Food Science and TechnologyDokument19 SeitenIntroduction to Food Science and TechnologyAisha DollNoch keine Bewertungen
- INTRODUCTION TO COMPASS SURVEYyDokument13 SeitenINTRODUCTION TO COMPASS SURVEYyCristiano RonaldoNoch keine Bewertungen
- CBSE Class 9 Biology Natural Resources Notes PDFDokument10 SeitenCBSE Class 9 Biology Natural Resources Notes PDFAbhishekNoch keine Bewertungen
- Numerical Model TurnerDokument696 SeitenNumerical Model TurnerChandan Kumar Ray100% (1)
- 08 HydroprocessingDokument38 Seiten08 HydroprocessingrciographyNoch keine Bewertungen
- Heparin-Containing Block Copolymers, Part II in Vitro and Ex Vivo Blood CompatibilityDokument12 SeitenHeparin-Containing Block Copolymers, Part II in Vitro and Ex Vivo Blood CompatibilityIvan ZkeyNoch keine Bewertungen
- Unit 3 Mass Transfer: StructureDokument22 SeitenUnit 3 Mass Transfer: StructureUdop CharlesNoch keine Bewertungen
- D Block ElementsDokument22 SeitenD Block Elementsketan kambleNoch keine Bewertungen
- A Review On Preparation of Low Cost Adhesive From Waste Materilal Using Citrus FruitsDokument10 SeitenA Review On Preparation of Low Cost Adhesive From Waste Materilal Using Citrus FruitsPRASHANT INGOLENoch keine Bewertungen
- Meta Doc TecnicalDokument9 SeitenMeta Doc TecnicalkarthidhulasiNoch keine Bewertungen
- Stress Strain Diagram for Ductile and Brittle MaterialsDokument15 SeitenStress Strain Diagram for Ductile and Brittle MaterialsWaqas Qureshi100% (5)
- Metal ReductionDokument11 SeitenMetal Reductiondeepthimahanthi sabithaNoch keine Bewertungen
- Chapter 14Dokument8 SeitenChapter 14nelaojNoch keine Bewertungen
- PN Junction Fabrication: Co-Ordinator DR Tarun ChaudharyDokument43 SeitenPN Junction Fabrication: Co-Ordinator DR Tarun ChaudharyBIYYAPU SAI VAMSINoch keine Bewertungen
- Lab ApparatusDokument10 SeitenLab ApparatuslizNoch keine Bewertungen
- Metco 54NS-1 (Aluminum Seal Coat) PDFDokument3 SeitenMetco 54NS-1 (Aluminum Seal Coat) PDFJ. BangjakNoch keine Bewertungen
- DPP 25C Goc Mesomeric 1686185793412Dokument3 SeitenDPP 25C Goc Mesomeric 1686185793412Aditya KumarNoch keine Bewertungen
- NQ Panelboards - NQ454L4C PDFDokument2 SeitenNQ Panelboards - NQ454L4C PDFdarniel quimbiulcoNoch keine Bewertungen
- Design of Tubular Reactor for Monochlorobenzene ProductionDokument2 SeitenDesign of Tubular Reactor for Monochlorobenzene ProductionRhea Joy C. MoralesNoch keine Bewertungen
- RecyclingPolicy Anovel3DPolymerBlendForFusedFilamentFabrication Earth2022 Full-Paper CompressedDokument5 SeitenRecyclingPolicy Anovel3DPolymerBlendForFusedFilamentFabrication Earth2022 Full-Paper CompressedShanya OrasutthikulNoch keine Bewertungen
- ASTM A295-98 (Obsolete)Dokument2 SeitenASTM A295-98 (Obsolete)techietaddyNoch keine Bewertungen
- Titrasi Asam Basa Dan Reaksi RedoksDokument40 SeitenTitrasi Asam Basa Dan Reaksi RedoksPutri RiduanNoch keine Bewertungen
- Liquid Waste Management in Food IndustriesDokument49 SeitenLiquid Waste Management in Food IndustriesGowrishankarNoch keine Bewertungen
- MTS Model 4340 SteelDokument36 SeitenMTS Model 4340 Steelb.banerjee.nz680075% (4)
- CathodoluminescenceDokument336 SeitenCathodoluminescenceJosé RamírezNoch keine Bewertungen
- Controlling the evaporator in urea productionDokument15 SeitenControlling the evaporator in urea productiontariq fareedNoch keine Bewertungen
- 41.1.28 AOAC Official Method 969.33 Fatty Acids in Oils and FatsDokument1 Seite41.1.28 AOAC Official Method 969.33 Fatty Acids in Oils and FatsNguyễn Khang LuânNoch keine Bewertungen