Beruflich Dokumente
Kultur Dokumente
Molecular Pathogenesis of Paroxysmal Nocturnal Haemoglobinuria
Hochgeladen von
Fra1312Originalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Molecular Pathogenesis of Paroxysmal Nocturnal Haemoglobinuria
Hochgeladen von
Fra1312Copyright:
Verfügbare Formate
Molecular Pathogenesis of Paroxysmal Nocturnal Haemoglobinuria
Taroh Kinoshita Introduction Paroxysmal nocturnal haemoglobinuria (PNH) is an acquired anaemia caused by complement-mediated haemolysis. Patients with PNH have abnormal red blood cells that are sensitive to complement due to the deficiency of cell surface complement regulatory proteins CD59 and decay-accelerating factor (DAF, CD55). The CD59/DAF-deficient red blood cells are lysed by complement slowly during physiologic low-level activation and rapidly during elevated activation caused by infections and other events. The affected red cells are of clonal origin and coexist with normal cells at a ratio which varies among patients. The CD59/DAF-deficient cell population appears also in various other haematopoietic lineages but not in nonhaematopoietic lineages, indicating that PNH is a haematopoietic stem cell disorder. CD59 and DAF are glycosylphosphatidylinositol (GPI)-anchored proteins whose cell surface expression is dependent on posttranslational attachment of the GPI glycolipid anchor (reviewed in 1,2). The deficiency of their surface expressions seen in PNH is caused by defective biosynthesis of the GPI anchor (reviewed in 3,4). At least seventeen GPI-anchored proteins, such as alkaline phosphatase and acetylcholinesterase, are known to be deficient on various haematopoietic cells.(3,4) A gene responsible for the deficiency of the GPI-anchor in PNH was cloned four years ago through a somatic genetic approach. Somatic Genetic Approach Towards a Gene Responsible for GPI-Anchor Deficiency in PNH Hyman and colleagues established many GPI anchor-deficient mutant cell lines from murine thymoma cells and grouped them into several complementation classes by somatic cell hybridization technique.(5) Biochemical analysis of glycolipids in these mutant cells of different complementation classes elucidated respective defective steps in the GPI biosynthesis pathway, in which at least eight steps are involved (reviewed in 2). We took advantage of this successful somatic genetic approach to determine a defective biosynthesis step in affected blood cells from patients. We established affected B lymphoblastoid cell lines from two patients that are deficient in the surface expressions of CD59 and DAF. We hybridized them by cell fusion to the classified mutant thymoma cells and found that PNH cell lines from both patients belong to complementation class A.(6) Cells of class A are defective in the first step of the biosynthesis of GPI, which is a transfer of N-acetylglucosamine (GlcNAc) to phosphatidylinositol (reviewed in 1,2). The PNH cell lines were also defective in the first step.(6) These results, confirmed by other groups (reviewed in 3,4), established that a class A mutant gene that participates in the first step of GPI biosynthesis is defective in the affected cells from many patients with PNH.
We further took a genetic approach to clone the gene responsible for the class A mutation and hence for GPI-anchor deficiency in PNH. We transfected a HeLa expression cDNA library into human B lymphoblastoid mutant cells of class A complementation group, concentrated cells that regained the surface expressions of CD59 and DAF by means of a cell-sorter, and recovered plasmids from them. We obtained a cDNA that restored synthesis of GPI anchor precursors in, and the surface expression of GPIanchored proteins on, class A mutant cells upon transfection, and termed the gene PIG-A (phosphatidylinositol glycan-class A).(7) As expected, PIG-A cDNA restored the surface expressions of CD59 and DAF on the affected B lymphoblastoid cells from the two patients.(8) The cells from one of the patients, female, transcribed only abnormal PIG-A mRNA, which lacked the entire exon 5. We found a responsible mutation, a T deletion within the 5' splice site of intron 5, in one of the alleles of PIG-A. This was a somatic mutation presumably in a hematopoietic stem cell because wild-type B lymphoblastoid cells derived from the same patient did not have it and because affected granulocytes also had it.(8) To understand the selective transcription of the mutant allele, we determined the chromosomal location of the PIG-A (8) gene and found that it resides on the short arm of the X-chromosome at Xp22.1. Since one of the X-chromosomes in female somatic cells is inactivated (X-chromosome inactivation or lyonization), the selective transcription of the mutant allele should be due to the presence of the mutant allele on the active and the normal allele on the inactive chromosomes. The X-chromosomal location of PIG-A, therefore, accounts for the phenotypic expression of a recessive somatic mutation. These results indicated that somatic mutation in PIG-A is responsible for GPI-anchor deficiency of affected cells from patients with PNH.(8) Structure and Function of the PIG-A Gene and Protein The PIG-A cDNA consists of 3589 bp and contains an open reading frame of 1452 bp that encodes a protein of 484 amino acids.(7) The PIG-A gene consists of six exons that span about 17 kbp.(9,10) Two alternative splice sites within exon 2 generate two shorter transcripts without a functional activity of PIG-A.(11,12) PIG-A is one of the three genesPIG-A, -H and -Cthat are necessary for synthesis of the first intermediate, N-acetylglucosaminyl phosphatidylinositol, in the endoplasmic reticulum (ER) (reviewed in 1,2). PIG-A protein is an ER transmembrane protein with its large amino-terminal portion on the cytoplasmic side and its small carboxy-terminal portion on the luminal side of the ER membrane (R. Watanabe, unpublished data). A region within the cytoplasmic portion has homology to a bacterial GlcNAc transferase, termed RfaK, that is involved in synthesis of lipopolysaccharide, suggesting that PIG-A is a catalytic subunit of the GlcNAc transferase.(13) We recently demonstrated that PIG-H is also an ER membrane protein facing the cytoplasm and that it forms a complex with PIG-A (R. Watanabe, unpublished data). Although the function of PIG-H is not known, these results are in agreement with the idea that the first intermediate is formed on the cytoplasmic side of the ER. We obtained PIG-C cDNA recently; however, its cellular localization has not been determined (N. Inoue, unpublished data).
Somatic Mutations of PIG-A in Patients with PNH The PIG-A gene has been analyzed in affected cell lines and/or granulocytes from more than 100 patients with PNH from American, European and Asian countries.(8,11,12,1423) Somatic mutations were found in most of them. In several patients, abnormality of PIG-A was demonstrated only at the mRNA level, presumably because responsible mutations reside within yet unidentified regulatory regions in PIG-A. It seems, therefore, that PIG-A is responsible for GPI-anchor deficiency in most if not all patients with PNH worldwide. In a few cases, attempts to determine the mutations have been unsuccessful due to the small fraction of affected cells. The X-chromosome location of PIG-A would account for this uniformity of the responsible gene among ten or so genes involved in biosynthesis of the GPI-anchor. As discussed above, one mutation in PIG-A would cause GPI-anchor deficiency for both males and females. In contrast, mutations should occur in both alleles in a single cell for autosomal genes. Since this event would be very rare, the X-chromosomal location of PIG-A would be the basis of its consistent involvement if all other GPI-synthesis genes are autosomal. In fact, the three other genesPIG-F, -H and -Blocalized to date are on autosomes.(24-26) Somatic mutations varied among patients and are small, 83 % involving only one or two bases. Of 122 mutations determined, 40 were single-base deletions and 41 were base substitutions. There were ten single-base insertions, sixteen 2-14 base deletions, eight 2-19 base insertions and one 4 kb deletion. Six cases were deletion/insertion. They were distributed widely in the coding regions and splice sites. There was no mutation clustering region, suggesting that mutations occur at random sites. Some patients had two to four mutant clones. Affected granulocytes usually contained a dominant clone, so only one mutation was identified in each of most patients by analysis of DNA and/or RNA from total granulocytes. In exceptional cases, two major clones of granulocytes coexist and both mutations were identified.(17) Mutations in minor clones have been successfully determined by analyzing lymphoid cell lines or colonies/bursts of CFU-GM or BFU-E. According to data so far reported, two or more clones have been identified in about 10% of patients. It is known that a quarter to a third of patients have a partially deficient red blood cell population and a completely deficient population. A mutation that causes partial deficiency must be different from that which causes complete deficiency, suggesting that those patients may have two mutant clones.(15) The real percentage of patients bearing multiple clones, therefore, would be much higher. Is the PIG-A Mutation Sufficient for PNH? There are two key events that lead to PNH: 1) Generation of a GPI-anchor deficient haematopoietic stem cell due to a somatic mutation of PIG-A, and 2) clonal expansion of that mutant and subsequent appearance of a GPI-anchor deficient red blood cell population. It is not clear whether mutation of PIG-A alone causes both GPI-anchor deficiency and clonal expansion or whether other factors are involved in clonal
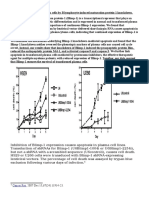
expansion. We addressed this issue by making GPI-anchor deficient mice.(26) We disrupted a mouse homologue of PIG-A, termed Pig-a,(13) in a male embryonic stem (ES) cell. This resulted in a loss of the surface expression of GPI-anchored proteins, showing that biosynthesis of the GPI-anchor was abolished due to the disruption of Pig-a.(27) We made chimaeric mice with this GPI-anchor deficient ES cell. Because of a lethal effect of GPI-anchor deficiency, high chimaerism was not achieved. Six of the chimaeras had 0.2 to 4% GPI-anchored protein-negative red blood cells three weeks after birth. If the loss of Pig-a function alone confers an ability to expand on haematopoietic stem cells, proportions of the GPI-anchored protein-negative red blood cells would increase with time. So we monitored them for five to seventeen months. They were constant or decreased until ten months, indicating that Pig-a mutation does not cause immediate expansion of the haematopoietic stem cells.(27) Therefore, other pathological or physiological factors may be necessary for the clonal expansion of GPI-anchor deficient cells. Interestingly, a proportion of the GPI-anchored protein-negative red blood cells in one of the six mice began to increase at twelve months of age from 4-5% and reached (27) 30% at seventeen months of age when the mouse died for an unknown reason. This mouse may have experienced other factors. If this is true, mice that harbor an inactivating mutation of Pig-a in haematopoietic stem cells may have a good chance to experience other factors leading to clonal expansion of the mutant. Conclusions and Perspectives Somatic mutation of PIG-A is responsible for the GPI-anchor deficiency in most if not all patients with PNH worldwide. Results with Pig-adeficient mice, however, suggested that other factor(s) may also be involved in the clonal expansion of the mutant haematopoietic cells. Identification of these factors is essential for a better understanding of the molecular basis of PNH. A mouse model would be useful for this. To obtain mice developing clonal expansion, many chimaeric mice should be generated. To circumvent a lethality caused by GPI-anchor deficiency in various tissues, use of conditional or tissuespecific knock out of Pig-a by means of Cre-loxP system should be effective. References 1. Stevens VL: Biosynthesis of glycosylphosphatidylinositol membrane anchors. Biochem J 310:361-370, 1995 2. Takeda J, Kinoshita T: GPI-anchor biosynthesis. Trends Biochem Sci 20:367-371, 1995 3. Kinoshita T, Inoue N, Takeda J: Defective glycosyl phosphatidylinositol anchor synthesis and paroxysmal nocturnal hemoglobinuria. Adv Immunol 60:57-103, 1995 4. Rosse WF, Ware RE: The molecular basis of paroxysmal nocturnal hemoglobinuria. Blood 86:3277-3286, 1995 5. Hyman R: Somatic genetic analysis of the expression of cell surface molecules. Trends Genet 4:5-8, 1988
6. Takahashi M, Takeda J, Hirose S, Hyman R, Inoue N, Miyata T, Ueda E, Kitani T, Medof ME, Kinoshita T: Deficient biosynthesis of N-acetylglucosaminyl phosphatidylinositol, the first intermediate of glycosyl phosphatidylinositol anchor biosynthesis, in cell lines established from patients with paroxysmal nocturnal hemoglobinuria. J Exp Med 177:517-521, 1993 7. Miyata T, Takeda J, Iida Y, Yamada N, Inoue N, Takahashi M, Maeda K, Kitani T, Kinoshita T: Cloning of PIG-A, a component in the early step of GPI-anchor biosynthesis. Science 259:1318-1320, 1993 8. Takeda J, Miyata T, Kawagoe K, Iida Y, Endo Y, Fujita T, Takahashi M, Kitani T, Kinoshita T: Deficiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell 73:703-711, 1993 9. Iida Y, Takeda J, Miyata T, Inoue N, Nishimura J, Kitani T, Maeda K, Kinoshita T: Characterization of genomic PIG-A gene: a gene for GPI-anchor biosynthesis and paroxysmal nocturnal hemoglobinuria. Blood 83:3126-3131, 1994 10. Bessler M, Hillmen P, Longo L, Luzzatto L, Mason PJ: Genomic organization of the X-linked gene (PIG-A) that is mutated in paroxysmal nocturnal haemoglobinuria and of a related autosomal pseudogene mapped to 12q21. Hum Mol Genet 3:751-757, 1994 11. Miyata T, Yamada N, Iida Y, Nishimura J, Takeda J, Kitani T, Kinoshita T: Abnormalities of PIG-A transcripts in granulocytes from patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med 330:249-255, 1994 12. Bessler M, Mason PJ, Hillmen P, Miyata T, Yamada N, Takeda J, Luzzatto L, Kinoshita T: Paroxysmal nocturnal haemoglobinuria (PNH) is caused by somatic mutations in the PIG-A gene. Embo J 13:110-117, 1994 13. Kawagoe K, Takeda J, Endo Y, Kinoshita T: Molecular cloning of murine Pig-a, a gene for GPI-anchor biosynthesis, and demonstration of interspecies conservation of its structure, function and gene locus. Genomics 23:566-574, 1994 14. Bessler M, Mason P, Hillmen P, Luzzatto L: Somatic mutations and cellular selection in paroxysmal nocturnal haemoglobinuria. Lancet 343:951-953, 1994 15. Bessler M, Mason PJ, Hillmen P, Luzzatto L: Mutations in the PIG-A gene causing partial deficiency of GPI-linked surface proteins (PNH II) in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol 87:863-866, 1994 16. Ware RE, Rosse WF, Howard TA: Mutations within the Pig-a gene in patients with paroxysmal nocturnal hemoglobinuria. Blood 83:2418-2422, 1994 17. Yamada N, Miyata T, Maeda K, Kitani T, Takeda J, Kinoshita T: Somatic mutations of the PIG-A gene found in Japanese patients with paroxysmal nocturnal hemoglobinuria. Blood 85:885-892, 1995 18. Ostendorf T, Nischan C, Schubert J, Grussenmeyer T, Scholz C, ZielinskaSkowronek M, Schmidt RE: Heterogeneous PIG-A mutations in different cell lineages in paroxysmal nocturnal hemoglobinuria. Blood 85:1640-1646, 1995 19. Pramoonjago P, Wanachiwanawin W, Chinprasertsak S, Pattanapanayasat K, Takeda J, Kinoshita T: Somatic mutations of PIG-A in Thai patients with paroxysmal nocturnal hemoglobinuria. Blood 86:1736-1739, 1995
20. Stafford HA, Nagarajan S, Weinberg JB, Medof ME: PIG-A, DAF and protooncogene expression in paroxysmal nocturnal haemoglobinuria-associated acute myelogenous leukaemia blasts. Br J Haematol 89:72-78, 1995 21. Nafa K, Mason PJ, Hillmen P, Luzzatto L, Bessler M: Mutations in the PIG-A gene causing paroxysmal nocturnal hemoglobinuria are mainly of the frameshift type. Blood 86:4650-4655, 1995 22. Nagarajan S, Brodsky RA, Young NS, Medof ME: Genetic defects underlying paroxysmal nocturnal hemoglobinuria that arises out of aplastic anemia. Blood 86:4656-4661, 1995 23. Nishimura J-I, Inoue N, Azenishi Y, Hirota T, Akaogi T, Shibano M, Kawagoe K, Ueda E, Machii T, Takeda J, Kinoshita T, Kitani T: Analysis of PIG-A gene in a patient who developed reciprocal translocation of chromosome 12 and paroxysmal nocturnal hemoglobinuria during follow-up of aplastic anemia. Am J Hematol 51:229-233, 1996 24. Ware RE, Howard TA, Kamitani T, Chang HM, Yeh ETH, Seldin MF: Chromosomal assignment of genes involved in glycosylphosphatidylinositol anchor biosynthesis: Implications for the pathogenesis of paroxysmal nocturnal hemoglobinuria. Blood 83:3753-3757, 1994 25. Ohishi K, Inoue N, Endo Y, Fujita T, Takeda J, Kinoshita T: Structure and chromosomal localization of the GPI-anchor synthesis gene PIG-F and its pseudogene YPIG-F. Genomics 29:804-807, 1995 26. Takahashi M, Inoue N, Ohishi K, Maeda Y, Nakamura N, Endo Y, Fujita T, Takeda J, Kinoshita T: PIG-B, a membrane protein of the endoplasmic reticulum with a large lumenal domain, is involved in transferring the third mannose of the GPI anchor. EMBO J, in press 27. Kawagoe K, Kitamura D, Okabe M, Taniuchi I, Ikawa M, Watanabe T, Kinoshita T, Takeda J: GPI-anchor deficient mice: Implications for clonal dominance of mutant cells in paroxysmal nocturnal hemoglobinuria. Blood 87:3600-3606, 1996
Das könnte Ihnen auch gefallen
- Genetic Linkage & Mapping (Article) - Khan AcademyDokument22 SeitenGenetic Linkage & Mapping (Article) - Khan Academy嘉雯吳Noch keine Bewertungen
- Fermentation FundamentalsDokument5 SeitenFermentation FundamentalsWade ColemanNoch keine Bewertungen
- 2.7 DNA Replication, Transcription, and TranslationDokument25 Seiten2.7 DNA Replication, Transcription, and TranslationJennNoch keine Bewertungen
- IB Biology Notes - 1 Working With DataDokument1 SeiteIB Biology Notes - 1 Working With DataWanda Wawa EvirhaNoch keine Bewertungen
- Introduction To Biotechnology PDFDokument39 SeitenIntroduction To Biotechnology PDFFitaSucia100% (1)
- Bioresources and Bioprocess in Biotechnology 2017Dokument442 SeitenBioresources and Bioprocess in Biotechnology 2017Angelynne Castro100% (1)
- Platelet Antigens and Antibody Detection: Original PaperDokument5 SeitenPlatelet Antigens and Antibody Detection: Original PaperstasirobertoNoch keine Bewertungen
- Molecular and Structural Biology of Factor IXDokument81 SeitenMolecular and Structural Biology of Factor IXALBERTOLPZNoch keine Bewertungen
- Pasq 2004 BloodDokument8 SeitenPasq 2004 BloodlillareinigerNoch keine Bewertungen
- Jcinvest00095 0208Dokument7 SeitenJcinvest00095 0208bilo47762Noch keine Bewertungen
- GprojectDokument10 SeitenGprojectlyraealpha941Noch keine Bewertungen
- PROTOTYPEDokument12 SeitenPROTOTYPEFrankenstein MelancholyNoch keine Bewertungen
- Up-Regulation of Luciferase Gene Expression With Antisense Oligonucleotides: Implications and Applications in Functional Assay DevelopmentDokument5 SeitenUp-Regulation of Luciferase Gene Expression With Antisense Oligonucleotides: Implications and Applications in Functional Assay DevelopmentFilip IlievskiNoch keine Bewertungen
- Hum. Mol. Genet.-2005-Benet-Pagès-385-90Dokument6 SeitenHum. Mol. Genet.-2005-Benet-Pagès-385-90daniel_geiger6597Noch keine Bewertungen
- A Novel Somatic K-Ras Mutation in Juvenile Myelomonocytic LeukemiaDokument2 SeitenA Novel Somatic K-Ras Mutation in Juvenile Myelomonocytic LeukemiamahanteshNoch keine Bewertungen
- Alterations in Lipid Metabolism Gene Expression and Abnormal Lipid Accumulation in Fibroblast Explant From Neuropathy PatientDokument12 SeitenAlterations in Lipid Metabolism Gene Expression and Abnormal Lipid Accumulation in Fibroblast Explant From Neuropathy PatientKrishnaNoch keine Bewertungen
- Jvetres 63 001Dokument6 SeitenJvetres 63 001José Hiram Sánchez GascaNoch keine Bewertungen
- Regression/Eradication of Gliomas in Mice by A Systemically-Deliverable ATF5 Dominant-Negative PeptideDokument13 SeitenRegression/Eradication of Gliomas in Mice by A Systemically-Deliverable ATF5 Dominant-Negative PeptideMarliCurcioNoch keine Bewertungen
- Hutchinson-Gilford Progeria Syndrome: Mini ReviewDokument7 SeitenHutchinson-Gilford Progeria Syndrome: Mini ReviewBrenda Argomedo CamposNoch keine Bewertungen
- Vockerodt Et Al-2008-The Journal of PathologyDokument10 SeitenVockerodt Et Al-2008-The Journal of PathologyBeatrice FacchiniNoch keine Bewertungen
- Bioinformatics Assignment: Medha Banerjee 1660091 IMTH-7 (A)Dokument11 SeitenBioinformatics Assignment: Medha Banerjee 1660091 IMTH-7 (A)Medha BanerjeeNoch keine Bewertungen
- 2011 SeifertDokument11 Seiten2011 SeifertNelson AlvarengaNoch keine Bewertungen
- Cancer Res 1998 Potter 3627 32Dokument7 SeitenCancer Res 1998 Potter 3627 32Frian LiaNoch keine Bewertungen
- Tugas Bu AniDokument23 SeitenTugas Bu AniYon-SyuhandaNoch keine Bewertungen
- Seminar On : Targeting Intrinsic Pathway of Apoptosis by A Naturalcompound For Anticancer TherapyDokument52 SeitenSeminar On : Targeting Intrinsic Pathway of Apoptosis by A Naturalcompound For Anticancer TherapyParvathiBaiNoch keine Bewertungen
- Green 2003Dokument6 SeitenGreen 200323501122Noch keine Bewertungen
- Pyruvate Kinase Deficiency and Malaria: Brief ReportDokument6 SeitenPyruvate Kinase Deficiency and Malaria: Brief ReportErlangga Perwira NegaraNoch keine Bewertungen
- The Role of Polymorphic Cytochrome P450 Gene CYP2B 2Dokument13 SeitenThe Role of Polymorphic Cytochrome P450 Gene CYP2B 2sherifref3atNoch keine Bewertungen
- Esofageal CancerDokument3 SeitenEsofageal CancerNaja HasnandaNoch keine Bewertungen
- tmpD7ED TMPDokument10 SeitentmpD7ED TMPFrontiersNoch keine Bewertungen
- Blimp MM Papers 3 31 13Dokument5 SeitenBlimp MM Papers 3 31 13api-216596948Noch keine Bewertungen
- Molmed 2011 00104Dokument8 SeitenMolmed 2011 00104HaveezhNoch keine Bewertungen
- J Immunol 2005 Hillion 5553 61Dokument10 SeitenJ Immunol 2005 Hillion 5553 61davdavdavdavdavdavdaNoch keine Bewertungen
- CD2BP2-GYF Interacts With The Spliceosomal Protein SMB - B' 1 (PDFDrive)Dokument31 SeitenCD2BP2-GYF Interacts With The Spliceosomal Protein SMB - B' 1 (PDFDrive)Blink SNoch keine Bewertungen
- Genetics Eview QuestionsDokument5 SeitenGenetics Eview QuestionsAmanda ParkNoch keine Bewertungen
- Arginine Deiminase As A Novel Therapy For Prostate Cancer Induces Autophagy and Caspase-Independent ApoptosisDokument9 SeitenArginine Deiminase As A Novel Therapy For Prostate Cancer Induces Autophagy and Caspase-Independent ApoptosisAvishekh SinhaNoch keine Bewertungen
- Fpi en Biologia MolecularDokument9 SeitenFpi en Biologia Molecularsofia barraganNoch keine Bewertungen
- Arn de InterferenciaDokument8 SeitenArn de InterferenciaRosita Aguirre FloresNoch keine Bewertungen
- Review Article On ImatinibDokument11 SeitenReview Article On ImatinibNathan ColleyNoch keine Bewertungen
- Letters: Gamma-Secretase Activating Protein Is A Therapeutic Target For Alzheimer's DiseaseDokument21 SeitenLetters: Gamma-Secretase Activating Protein Is A Therapeutic Target For Alzheimer's DiseaseFoo BarNoch keine Bewertungen
- tmp48C6 TMPDokument11 Seitentmp48C6 TMPFrontiersNoch keine Bewertungen
- g6pd - An Old Bottle With New WineDokument7 Seiteng6pd - An Old Bottle With New Winecutegal88Noch keine Bewertungen
- Development of Anti-Peptide Polyclonal Antibodies Raised Against Immunogenic Sequences of Protein Encoded by Fj194940.1 GeneDokument9 SeitenDevelopment of Anti-Peptide Polyclonal Antibodies Raised Against Immunogenic Sequences of Protein Encoded by Fj194940.1 GeneijsidonlineinfoNoch keine Bewertungen
- Chromosomal Aberrations Induced by 5-Azacytidine Combined With VP-16 (Etoposide) in CHO-K1 and XRS-5 Cell LinesDokument16 SeitenChromosomal Aberrations Induced by 5-Azacytidine Combined With VP-16 (Etoposide) in CHO-K1 and XRS-5 Cell LinesAry AguiarNoch keine Bewertungen
- A Chemical Biology Screen Identifies Glucocorticoids That Regulate C - 2007 - BLDokument8 SeitenA Chemical Biology Screen Identifies Glucocorticoids That Regulate C - 2007 - BLTareeqanwar MohammedNoch keine Bewertungen
- The Antitumor Activity of Tea Is Attributed To Its Capacity To Mediate Signaling Pathways and Regulate CellsDokument3 SeitenThe Antitumor Activity of Tea Is Attributed To Its Capacity To Mediate Signaling Pathways and Regulate CellsvandanaNoch keine Bewertungen
- SCARDUSconf FfffinalDokument6 SeitenSCARDUSconf Ffffinalbucajselma20Noch keine Bewertungen
- Identification and Molecular Analysis of 17 Novel Variants of HMBSDokument14 SeitenIdentification and Molecular Analysis of 17 Novel Variants of HMBSdivillamarinNoch keine Bewertungen
- DHH-RHEBL1 Fusion Transcript A Novel Recurrent AML Ricardo, 2013Dokument9 SeitenDHH-RHEBL1 Fusion Transcript A Novel Recurrent AML Ricardo, 2013mrkhprojectNoch keine Bewertungen
- Zhou (2012) Usefulness of CD11a and CD18 in Flow Cytometric Immunophenotypic Analysis For Diagnosis of Acute Promyelocytic LeukemiaDokument7 SeitenZhou (2012) Usefulness of CD11a and CD18 in Flow Cytometric Immunophenotypic Analysis For Diagnosis of Acute Promyelocytic LeukemiaJosué Cristhian Del Valle HornaNoch keine Bewertungen
- A Rare Case of Factor X Deficiency AssociatedDokument16 SeitenA Rare Case of Factor X Deficiency AssociatedRoss Mark PerandosNoch keine Bewertungen
- A New Recurrent IGH@ Translocation Involving ID4 in B-Cell Precursor Acute Lymphoblastic Leukemia (BCP-ALL)Dokument6 SeitenA New Recurrent IGH@ Translocation Involving ID4 in B-Cell Precursor Acute Lymphoblastic Leukemia (BCP-ALL)Dev SubbukuttiNoch keine Bewertungen
- Isolation and Characterization of Human Erythrocytes Inhibits Lysis of The Erythrocytes Paroxysmal HemoglobinuriaDokument11 SeitenIsolation and Characterization of Human Erythrocytes Inhibits Lysis of The Erythrocytes Paroxysmal HemoglobinuriaKurniawatiNoch keine Bewertungen
- Retroviral-Mediated Very-Long-Chain Fatty Acid Metabolism in Adrenoleukodystrophy FibroblastsDokument5 SeitenRetroviral-Mediated Very-Long-Chain Fatty Acid Metabolism in Adrenoleukodystrophy FibroblastsmdquevedoNoch keine Bewertungen
- (66-2000) Downregulation of b1 Integrins by Ebola Virus Glycoprotein Implication For Virus EntryDokument7 Seiten(66-2000) Downregulation of b1 Integrins by Ebola Virus Glycoprotein Implication For Virus Entryfabian cotacioNoch keine Bewertungen
- Rbl Gamma Chain Deficient Cell Line-Model For Assessing Α And Γ Subunit InteractionsDokument19 SeitenRbl Gamma Chain Deficient Cell Line-Model For Assessing Α And Γ Subunit InteractionsAmirNoch keine Bewertungen
- Aberrations of MYC Are A Common Event in B-Cell Prolymphocytic LeukemiaDokument9 SeitenAberrations of MYC Are A Common Event in B-Cell Prolymphocytic LeukemiaRafa AssidiqNoch keine Bewertungen
- Molecular Basis of AMP Deaminase Deficiency in Skeletal MuscleDokument5 SeitenMolecular Basis of AMP Deaminase Deficiency in Skeletal MuscleamraovcinaNoch keine Bewertungen
- Myeloid NeoplasiaDokument10 SeitenMyeloid NeoplasiaivssonNoch keine Bewertungen
- N-Acetyl-: - Glucosaminylphosphatidylinositol De-N-acetylase From Entamoeba HistolyticaDokument7 SeitenN-Acetyl-: - Glucosaminylphosphatidylinositol De-N-acetylase From Entamoeba HistolyticaDr. Mohd Ashraf KhanNoch keine Bewertungen
- GM-CSF: Biology and Appropriate Clinical Applications: Graham J. LieschkeDokument12 SeitenGM-CSF: Biology and Appropriate Clinical Applications: Graham J. LieschkePercy Urbina GradosNoch keine Bewertungen
- Artículo 1Dokument10 SeitenArtículo 1AoriwishNoch keine Bewertungen
- Glucose-6-Phosphate Dehydrogenase DeficiencyDokument3 SeitenGlucose-6-Phosphate Dehydrogenase DeficiencyNoca AriantiNoch keine Bewertungen
- tmpB2BC TMPDokument6 SeitentmpB2BC TMPFrontiersNoch keine Bewertungen
- Regular Article: Ph-Like Acute Lymphoblastic Leukemia: A High-Risk Subtype in AdultsDokument10 SeitenRegular Article: Ph-Like Acute Lymphoblastic Leukemia: A High-Risk Subtype in AdultsKe XuNoch keine Bewertungen
- Inotropes 2014. Journal of The American College of CardiologyDokument10 SeitenInotropes 2014. Journal of The American College of CardiologyFra1312Noch keine Bewertungen
- Shock Septico EvidenciaDokument8 SeitenShock Septico EvidenciaFra1312Noch keine Bewertungen
- Ertapenem Review of New CarbapenemDokument17 SeitenErtapenem Review of New CarbapenemFra1312Noch keine Bewertungen
- Community-Acquired Pneumonia: Role of Atypical Organisms: R. Cosentini, P. Tarsia, F. Blasi, E. Roma, L. AllegraDokument8 SeitenCommunity-Acquired Pneumonia: Role of Atypical Organisms: R. Cosentini, P. Tarsia, F. Blasi, E. Roma, L. AllegraFra1312Noch keine Bewertungen
- Enterobacteriaceae: Biochemical ReactionsDokument20 SeitenEnterobacteriaceae: Biochemical Reactionslindaprihastiwi100% (1)
- BioTechCorp-Annual Report 2011Dokument150 SeitenBioTechCorp-Annual Report 2011drbanana84Noch keine Bewertungen
- DeoxyribonucleaseDokument4 SeitenDeoxyribonucleaseAndiMuhYasserNoch keine Bewertungen
- Cell DivisionDokument5 SeitenCell DivisionEloisa MadrilenoNoch keine Bewertungen
- Vtu SylDokument114 SeitenVtu SylIshwar ChandraNoch keine Bewertungen
- Paraphrasing and Summarizing ActivityDokument2 SeitenParaphrasing and Summarizing ActivityMISLIIIFLFIOOOOOONoch keine Bewertungen
- SM ProjectDokument6 SeitenSM ProjectSiddharth SrivastavNoch keine Bewertungen
- Dneasy Plant Mini Kit and Dneasy Plant Maxi Kit Handbook: For Dna Isolation From Plant TissueDokument28 SeitenDneasy Plant Mini Kit and Dneasy Plant Maxi Kit Handbook: For Dna Isolation From Plant TissueEl-Agamy ProbeNoch keine Bewertungen
- Bioplastics - As Green Alternative To PlasticsDokument3 SeitenBioplastics - As Green Alternative To PlasticsVasantha PrasathNoch keine Bewertungen
- Animal BiotechnologyDokument13 SeitenAnimal Biotechnologyaisha meharNoch keine Bewertungen
- Hema 1Dokument25 SeitenHema 1Mark SyNoch keine Bewertungen
- 6-Kidney Bioengineering Dr. Nathaniel Dugos PDFDokument14 Seiten6-Kidney Bioengineering Dr. Nathaniel Dugos PDFNora O. GamoloNoch keine Bewertungen
- Cut-Off Points For Admission Under The Government Sponsorship Scheme For The Academic Year 2015/2016.Dokument4 SeitenCut-Off Points For Admission Under The Government Sponsorship Scheme For The Academic Year 2015/2016.The Campus Times100% (1)
- Abzyme Files Patent Application For Self-Diversifying Antibody PlatformDokument2 SeitenAbzyme Files Patent Application For Self-Diversifying Antibody PlatformPR.comNoch keine Bewertungen
- Genomics Transgenics Molecular Breeding PDFDokument139 SeitenGenomics Transgenics Molecular Breeding PDFJose ODNoch keine Bewertungen
- Relative Quantitation Using Comparative C Getting Started GuideDokument99 SeitenRelative Quantitation Using Comparative C Getting Started Guidesalman672003Noch keine Bewertungen
- Lectinas de Ajo Rol InsecticidaDokument8 SeitenLectinas de Ajo Rol Insecticidajose gpeNoch keine Bewertungen
- Transient Receptor Potential Channels and MechanosensationDokument28 SeitenTransient Receptor Potential Channels and MechanosensationSamuel BeaumontNoch keine Bewertungen
- Vandamme Vitamins and Growth FactorsDokument439 SeitenVandamme Vitamins and Growth FactorsAlexandreSidantNoch keine Bewertungen
- Modern BiotechnologyDokument23 SeitenModern BiotechnologyAnderson Chandra 1422031Noch keine Bewertungen
- Experimental and Molecular Pathology: SciencedirectDokument8 SeitenExperimental and Molecular Pathology: SciencedirectrafaelaqNoch keine Bewertungen
- Curriculum Vitae: G.SethuramanDokument4 SeitenCurriculum Vitae: G.SethuramanSethuNoch keine Bewertungen
- DNA Plasmid IsolationDokument3 SeitenDNA Plasmid IsolationMahtab AhmadNoch keine Bewertungen
- B. Tech. (Biotechnology) : 2011 SyllabusDokument5 SeitenB. Tech. (Biotechnology) : 2011 SyllabusSwaminathan Balasubramanian BNoch keine Bewertungen