Beruflich Dokumente
Kultur Dokumente
AP301 Modern Physics I: Prof. Daniel S. P. Lau Daniel - Lau@polyu - Edu.hk Tel: 2766 5679 Office: CD622
Hochgeladen von
doldolzzOriginalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
AP301 Modern Physics I: Prof. Daniel S. P. Lau Daniel - Lau@polyu - Edu.hk Tel: 2766 5679 Office: CD622
Hochgeladen von
doldolzzCopyright:
Verfügbare Formate
AP301 Modern Physics I
Prof. Daniel S. P. Lau
daniel.lau@polyu.edu.hk
Tel: 2766 5679
Office: CD622
1
Lecture/Tutorial
Announcements
2
Tutorial class will begin on week 3 (12-16 Sept)
Tutorial groups (TUT003 and TUT004) on
Wednesday at 3:30pm, please note that the
venue has been changed to Y502.
A open-book quiz will be given by a tutor
during the lecture on 20 Sept.
Lecture on 21 Sept will be cancelled.
Tutorial class on 21 Sept will be cancelled.
Students from Tutorial group TUT004, please
attend make-up tutorial on 23 Sept at 6:30pm at
CD620.
Blackboard e-learning platform
http://learn.polyu.edu.hk
Course materials can be found in the course
site.
Please check the course site regularly to read
the announcements related to the course.
3
Contents - 1
Introduction to quantum physics
Blackbody Radiation
The Photoelectric Effect
The Compton Effect
Photons and Electromagnetic Waves
The Wave Properties of Particles
The Quantum Particle
The Double-slit Experiment Revisited
The Uncertainty Principle
4
Contents - 2
Quantum Mechanics
An Interpretation of Quantum Mechanics
The Quantum Particle Under Boundary
Conditions
The Schrdinger Equation
A Particle in a Well of Finite Height
Tunneling Through a Potential Energy Barrier
Applications of Tunneling
The Simple Harmonic Oscillator
5
Contents - 3
Atomic Physics
Atomic Spectra of Gases
Early Models of the Atom
Bohrs Model of the Hydrogen Atom
The Quantum Model of the Hydrogen Atom
The Wave Function for Hydrogen
Physical Interpretation of the Quantum Numbers
The Exclusion Principle and the Periodic Table
More on Atomic Spectra: Visible and X-Ray
Spontaneous and Stimulated Transition
Lasers
6
Contents - 4
Molecules and Solids
Molecular Bonds
Energy States and Spectra of Molecules
Bonding in Solids
Free-Electron Theory of Metals
Band Theory of Solids
Electrical Conduction in Metals, Insulators and
Semiconductors
Semiconductor Devices
Superconductivity
7
Textbook
Physics for Scientists and Engineers with
Modern Physics (Eighth Edition),
Jewett/Serway, Cengage Learning 2010.
8
Learning Outcomes - 1
articulate the experimental basis for attributing
particle properties to waves and wave properties
to particles; elaborate on the de Broglie theory of
matter waves; apply Heisenbergs uncertainty
principle to simple systems;
elaborate on the various forms of Schrdingers
equation and identify the meaning of each term in
the equation(s); solve Schrdingers equation for
the problem of particle in a box;
9
Learning Outcomes - 2
solve problems involving tunneling and
harmonic oscillator;
describe the scientific ideas behind the
historical atomic models and recognize and
justify the various modifications of classical
ideas as new experimental evidences
emerged; use Bohrs semiclassical model to
interpret energy levels and spectra and
recognize the limitations of the model;
10
Learning Outcomes - 3
explain the physical significance of the
quantum atomic model;
use a free-electron model to explain electrical
conduction in metals;
explain the electrical conduction in metals,
insulators and semiconductors; and
solve simple problems involving
semiconductors devices.
11
Assessments
Test (25%)
2 tests tentatively on week 6 and week 11
Quizzes and Exercise (10%)
Weekly quizzes (Wednesday)
Tutorial questions every two weeks
Clicker questions
Exam (65%)
12
Chapter 40
Introduction to
Quantum Physics
13
Need for Quantum Physics
Problems remained from classical mechanics that
relativity didnt explain
Attempts to apply the laws of classical physics to
explain the behavior of matter on the atomic scale
were consistently unsuccessful
Problems included:
Blackbody radiation
The electromagnetic radiation emitted by a heated object
Photoelectric effect
Emission of electrons by an illuminated metal
14
Quantum Mechanics
Revolution
Between 1900 and 1930, another revolution
took place in physics
A new theory called quantum mechanics was
successful in explaining the behavior of
particles of microscopic size
The first explanation using quantum theory
was introduced by Max Planck
Many other physicists were involved in other
subsequent developments
15
Quantum physics (video)
16
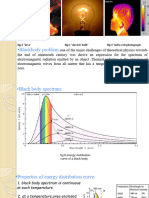
Blackbody Radiation
An object at any temperature is known to
emit thermal radiation
Characteristics depend on the temperature and
surface properties
The thermal radiation consists of a continuous
distribution of wavelengths from all portions of the
em spectrum
17
Blackbody Radiation, cont.
At room temperature, the wavelengths of the
thermal radiation are mainly in the infrared region
As the surface temperature increases, the
wavelength changes
It will glow red and eventually white
The basic problem was in understanding the
observed distribution in the radiation emitted by a
black body
Classical physics didnt adequately describe the observed
distribution
18
Blackbody Radiation, final
A black body is an ideal system that absorbs
all radiation incident on it
The electromagnetic radiation emitted by a
black body is called blackbody radiation
19
Blackbody Approximation
A good approximation of a
black body is a small hole
leading to the inside of a
hollow object
The hole acts as a perfect
absorber
The nature of the radiation
leaving the cavity through
the hole depends only on
the temperature of the
cavity
20
Blackbody Experiment Results
The total power of the emitted radiation increases
with temperature
Stefans law:
= oAeT
4
(40.1)
Stefan-Boltzmann constant equal to 5.67 x 10
-8
W/m
2
K
4
,
A is surface area of the object, e is the emissivity of the
surface. For a black body, e = 1 exactly.
The peak of the wavelength distribution shifts to
shorter wavelengths as the temperature increases
Wiens displacement law
max
T = 2.898 x 10
-3
m.K (40.2)
21
Intensity of Blackbody
Radiation, Summary
The intensity increases
with increasing
temperature
The amount of radiation
emitted increases with
increasing temperature
The area under the curve
The peak wavelength
decreases with
increasing temperature
22
Active Figure 40.3
Use the active figure to
adjust the temperature
of the blackbody
Study the emitted
radiation
PLAY
ACTIVE FIGURE
23
Quiz 40.1
Figure shows two stars in the constellation Orion.
Betelgeuse appears to glow red, whereas Rigel looks
blue in color. Which star has a higher surface
temperature?
a) Betelgeuse
b) Rigel
c) both the same
d) impossible to determine
24
Rayleigh-Jeans Law
An early classical attempt to explain
blackbody radiation was the Rayleigh-Jeans
law
At long wavelengths, the law matched
experimental results fairly well
( )
4
2
I ,
B
ck T
T
=
25
(40.3)
Rayleigh-Jeans Law, cont.
At short wavelengths, there
was a major disagreement
between the Rayleigh-
Jeans law and experiment
This mismatch became
known as the ultraviolet
catastrophe
You would have infinite
energy as the wavelength
approaches zero
26
Max Planck
1858 1947
German physicist
Introduced the concept
of quantum of action
In 1918 he was
awarded the Nobel
Prize for the discovery
of the quantized nature
of energy
27
Max Planck (Video)
28
Plancks Theory of Blackbody
Radiation
In 1900 Planck developed a theory of
blackbody radiation that leads to an equation
for the intensity of the radiation
This equation is in complete agreement with
experimental observations
He assumed the cavity radiation came from
atomic oscillations in the cavity walls
Planck made two assumptions about the
nature of the oscillators in the cavity walls
29
Plancks Assumption, 1
The energy of an oscillator can have only
certain discrete values E
n
E
n
= nh
n is a positive integer called the quantum number
is the frequency of oscillation
h is Plancks constant
This says the energy is quantized
Each discrete energy value corresponds to a
different quantum state
Each quantum state is represented by the quantum
number, n.
30
(40.4)
Plancks Assumption, 2
The oscillators emit or absorb energy when
making a transition from one quantum state
to another
The entire energy difference between the initial
and final states in the transition is emitted or
absorbed as a single quantum of radiation
An oscillator emits or absorbs energy only when it
changes quantum states
The energy carried by the quantum of radiation is
E = h
31
(40.5)
Energy-Level Diagram
An energy-level diagram
shows the quantized energy
levels and allowed
transitions
Energy is on the vertical
axis
Horizontal lines represent
the allowed energy levels
The double-headed arrows
indicate allowed transitions
32
More About Plancks Model
The average energy of a wave is the average
energy difference between levels of the
oscillator, weighted according to the
probability of the wave being emitted
This weighting is described by the Boltzmann
distribution law and gives the probability of a
state being occupied as being proportional to
where E is the energy of the state
B
E k T
e
33
Plancks
Model,
Graph
34
Active Figure 40.7
Use the active figure to
investigate the energy
levels
Observe the emission
of radiation of different
wavelengths
PLAY
ACTIVE FIGURE
35
Plancks Wavelength
Distribution Function
Planck generated a theoretical expression for
the wavelength distribution
h = 6.626 x 10
-34
J.s
h is a fundamental constant of nature
( )
( )
2
5
2
1
I ,
B
hc k T
hc
T
e
=
36
(40.6)
(40.7)
Plancks Wavelength
Distribution Function, cont.
At long wavelengths, Plancks equation
reduces to the Rayleigh-Jeans expression
At short wavelengths, it predicts an
exponential decrease in intensity with
decreasing wavelength
This is in agreement with experimental results
37
Einstein and Plancks Results
Einstein rederived Plancks results by
assuming the oscillations of the
electromagnetic field were themselves
quantized.
In other words, Einstein proposed that
quantization is a fundamental property of light
and other electromagnetic radiation.
This led to the concept of photons.
38
Example 40.1
Thermal Radiation from Different Objects
(A) Find the peak wavelength of the blackbody
radiation emitted by the human body when
the skin temperature is 35 C.
(B) Find the peak wavelength of the blackbody
radiation emitted by the tungsten filament of
a lightbulb, which operates at 2000 K.
(C) Find the peak wavelength of the blackbody
radiation emitted by the Sun, which has a
surface temperature of approximately 5800
K.
39
Example 40.1
40
Example 40.2
The quantized oscillator
A 2.0 kg block is attached to a massless spring
that has a force constant of k = 25 N/m. The spring
is stretched 0.4 m from its equilibrium position and
released from rest.
(A) Find the total energy of the system and the
frequency of oscillation according to classical
calculations.
(B) Assuming the energy of the oscillator is
quantized, find the quantum number n for the
system oscillating with this amplitude.
41
42
Example 40.2
Max Planck (video part 2)
43
Photoelectric Effect
The photoelectric effect occurs when light
incident on certain metallic surfaces causes
electrons to be emitted from those surfaces
The emitted electrons are called photoelectrons
They are no different than other electrons.
The name is given because of their ejection from a
metal by light in the photoelectric effect
44
Photoelectric effect (video)
45
Photoelectric Effect Apparatus
When the tube is kept in the
dark, the ammeter reads
zero
When plate E is illuminated
by light having an
appropriate wavelength, a
current is detected by the
ammeter
The current arises from
photoelectrons emitted from
the negative plate and
collected at the positive
plate
46
Active Figure 40.9
Use the active figure to
vary frequency or place
voltage
Observe the motion of
the electrons
PLAY
ACTIVE FIGURE
47
Photoelectric Effect, Results
At large values of AV, the
current reaches a maximum
value
All the electrons emitted at
E are collected at C
The maximum current
increases as the intensity of
the incident light increases
When AV is negative, the
current drops
When AV is equal to or more
negative than AV
s
, the
current is zero
48
Photoelectric Effect Feature 1
Dependence of photoelectron kinetic energy on light
intensity
Classical Prediction
Electrons should absorb energy continually from the
electromagnetic waves
As the light intensity incident on the metal is increased, the
electrons should be ejected with more kinetic energy
Experimental Result
The maximum kinetic energy is independent of light
intensity
The maximum kinetic energy is proportional to the stopping
potential (AV
s
)
49
Photoelectric Effect Feature 2
Time interval between incidence of light and ejection
of photoelectrons
Classical Prediction
At low light intensities, a measurable time interval should
pass between the instant the light is turned on and the time
an electron is ejected from the metal
This time interval is required for the electron to absorb the
incident radiation before it acquires enough energy to
escape from the metal
Experimental Result
Electrons are emitted almost instantaneously, even at very
low light intensities
50
Photoelectric Effect Feature 3
Dependence of ejection of electrons on light
frequency
Classical Prediction
Electrons should be ejected at any frequency as long as the
light intensity is high enough
Experimental Result
No electrons are emitted if the incident light falls below
some cutoff frequency,
c
The cutoff frequency is characteristic of the material being
illuminated
No electrons are ejected below the cutoff frequency
regardless of intensity
51
Photoelectric Effect Feature 4
Dependence of photoelectron kinetic energy
on light frequency
Classical Prediction
There should be no relationship between the
frequency of the light and the electric kinetic energy
The kinetic energy should be related to the intensity of
the light
Experimental Result
The maximum kinetic energy of the photoelectrons
increases with increasing light frequency
52
Photoelectric Effect Features,
Summary
The experimental results contradict all four
classical predictions
Einstein extended Plancks concept of
quantization to electromagnetic waves
All electromagnetic radiation can be considered
a stream of quanta, now called photons
Each photon has an energy E and moves at the
speed of light in a vacuum.
E = h
A photon of incident light gives all its energy h
to a single electron in the metal
53
Quiz 40.2
While standing outdoors one evening, you are exposed to the following
four types of electromagnetic radiation: yellow light from a sodium street
lamp, radio waves from an AM radio station, radio waves from an FM
radio station, and microwaves from an antenna of a communications
system. Rank these types of waves in terms of increasing photon energy,
lowest first.
a) sodium light, AM radio, FM radio, microwaves
b) AM radio, FM radio, microwaves, sodium light
c) microwaves, sodium light, AM radio, FM radio
d) FM radio, AM radio, sodium light, microwaves
e) none of the above
54
Photoelectric Effect, Work
Function
Electrons ejected from the surface of the
metal and not making collisions with other
metal atoms before escaping possess the
maximum kinetic energy K
max
K
max
= h
is called the work function
The work function represents the minimum energy
with which an electron is bound in the metal
55
(40.9)
Some Work
Function
Values
56
Photon Model Explanation of
the Photoelectric Effect
Dependence of photoelectron kinetic energy
on light intensity
K
max
is independent of light intensity
K depends on the light frequency and the work
function
Time interval between incidence of light and
ejection of the photoelectron
Each photon can have enough energy to eject an
electron immediately
57
Photon Model Explanation of
the Photoelectric Effect, cont.
Dependence of ejection of electrons on light
frequency
There is a failure to observe photoelectric effect
below a certain cutoff frequency, which indicates
the photon must have more energy than the work
function in order to eject an electron
Without enough energy, an electron cannot be
ejected, regardless of the fact that many photons
per unit time are incident on the metal in a very
intense light beam.
58
Photon Model Explanation of
the Photoelectric Effect, final
Dependence of photoelectron kinetic energy
on light frequency
Since K
max
= h
A photon of higher frequency carries more
energy.
A photoelectron is ejected with higher kinetic energy.
Once the energy of the work function is exceeded
There is a linear relationship between the kinetic
energy and the frequency
59
Cutoff Frequency
The lines show the
linear relationship
between K and
The slope of each line
is h
The x-intercept is the
cutoff frequency
This is the frequency
below which no
photoelectrons are
emitted
60
Cutoff Frequency and
Wavelength
The cutoff frequency is related to the work
function through
c
= / h
The cutoff frequency corresponds to a cutoff
wavelength
Wavelengths greater than
c
incident on a
material having a work function do not
result in the emission of photoelectrons
c
c
c hc
= =
61
(40.10)
Quiz 40.3
62
Consider one of the curves in Active Figure 40.10.
Suppose the intensity of the incident light is held fixed
but its frequency is increased. The stopping potential
in Active Figure 40.10:
Active Figure 40.10
a) remains fixed.
b) moves to the right.
c) moves to the left.
Example 40.3
The photoelectric effect for sodium
A sodium surface is illuminated with light
having a wavelength of 300 nm. The work
function for sodium metal is 2.46 eV.
(A)Find the maximum kinetic energy of the
ejected photoelectrons.
(B)Find the cutoff wavelength
c
for sodium.
63
Arthur Holly Compton
1892 1962
American physicist
Director of the lab at
the University of
Chicago
Discovered the
Compton Effect
Shared the Nobel Prize
in 1927
64
The Compton Effect,
Introduction
Compton and Debye extended with Einsteins
idea of photon momentum
The two groups of experimenters
accumulated evidence of the inadequacy of
the classical wave theory
The classical wave theory of light failed to
explain the scattering of x-rays from electrons
65
Compton Effect, Classical
Predictions
According to the classical theory, em waves
incident on electrons should:
Have radiation pressure that should cause the
electrons to accelerate
Set the electrons oscillating
There should be a range of frequencies for the
scattered electrons
66
Compton Effect, Observations
Comptons experiments
showed that, at any
given angle, only one
frequency of radiation is
observed
67
Compton Effect, Explanation
The results could be explained by treating the
photons as point-like particles having energy h
and momentum h / c.
Assume the energy and momentum of the
isolated system of the colliding photon-
electron are conserved
This scattering phenomena is known as the
Compton effect
68
Compton Shift Equation
The graphs show the
scattered x-ray for
various angles
The shifted peak, is
caused by the
scattering of free
electrons
This is called the
Compton shift equation
( )
1 ' cos
o
e
h
m c
=
69
(40.11)
Compton Wavelength
The factor h/m
e
c in the equation is called the
Compton wavelength and is
The unshifted wavelength,
o
, is caused by x-
rays scattered from the electrons that are
tightly bound to the target atoms
0002 43 nm
C
e
h
m c
= = .
70
m
e
= 9.11 10
-31
kg
Quiz 40.4
71
For any given scattering angle , Equation 40.11, ,
gives the same value for the Compton shift for any wavelength.
Keeping that in mind, for which of the following types of radiation
is the fractional shift in wavelength at a given scattering angle the
largest?
( )
c m
h
e
cos 1 '
0
=
a) radio waves
b) microwaves
c) visible light
d) x-rays
Example 40.4
Compton scattering at 45
X-rays of wavelength
0
= 0.200 000 nm are
scattered from a block of material. The
scattered x-rays are observed at an angle of
45.0 to the incident beam. Calculate their
wavelength.
72
Photons and Waves Revisited
Some experiments are best explained by the photon
model
Some are best explained by the wave model
We must accept both models and admit that the true
nature of light is not describable in terms of any single
classical model
The particle model and the wave model of light
complement each other.
A complete understanding of the observed behavior of
light can be attained only if the two models are combined
in a complementary matter.
73
Louis de Broglie
1892 1987
French physicist
Originally studied
history
Was awarded the
Nobel Prize in 1929 for
his prediction of the
wave nature of
electrons
74
Wave Properties of Particles
Louis de Broglie postulated that because
photons have both wave and particle
characteristics, perhaps all forms of matter
have both properties
The de Broglie wavelength of a particle is
h h
p mu
= =
75
(40.15)
Frequency of a Particle
In an analogy with photons, de Broglie
postulated that a particle would also have a
frequency associated with it
These equations present the dual nature of
matter
Particle nature, p and E
Wave nature, and
E
h
=
76
(40.16)
Quiz 40.5
77
An electron and a proton both moving at nonrelativistic
speeds have the same de Broglie wavelength. Which of
the following quantities are also the same for the two
particles?
a) speed
b) kinetic energy
c) momentum
d) frequency
Complementarity
The principle of complementarity states
that the wave and particle models of either
matter or radiation complement each other
Neither model can be used exclusively to
describe matter or radiation adequately
78
Davisson-Germer Experiment
If particles have a wave nature, then under
the correct conditions, they should exhibit
diffraction effects
Davisson and Germer measured the
wavelength of electrons
This provided experimental confirmation of
the matter waves proposed by de Broglie
79
Example 40.5
Wavelength for microscopic and
Macroscopic objects
(A) Calculate the de Broglie wavelength for an
electron (m
e
= 9.11 10
-31
kg) moving at
1.00 10
7
m/s.
(B) A rock of mass 50 g is thrown with a speed
of 40 m/s. What is its de Broglie
wavelength?
80
81
Wave Properties of Particles
Mechanical waves have materials that are
waving and can be described in terms of
physical variables.
A string may be vibrating.
Sound waves are produced by molecules of a
material vibrating.
Electromagnetic waves are associated with
electric and magnetic fields.
Waves associated with particles cannot be
associated with a physical variable.
82
Electron Microscope
The electron microscope relies on
the wave characteristics of
electrons
Shown is a transmission electron
microscope
Used for viewing flat, thin
samples
The electron microscope has a
high resolving power because it
has a very short wavelength
Typically, the wavelengths of the
electrons are about 100 times
shorter than that of visible light
83
Electron microscopy (video)
84
Quantum Particle
The quantum particle is a new model that is
a result of the recognition of the dual nature
Entities have both particle and wave
characteristics
We must choose one appropriate behavior in
order to understand a particular phenomenon
85
Ideal Particle vs. Ideal Wave
An ideal particle has zero size
Therefore, it is localized in space
An ideal wave has a single frequency and is
infinitely long
Therefore,it is unlocalized in space
A localized entity can be built from infinitely
long waves
86
Particle as a Wave Packet
Multiple waves are superimposed so that one of its
crests is at x = 0
The result is that all the waves add constructively at
x = 0
There is destructive interference at every point
except x = 0
The small region of constructive interference is
called a wave packet
The wave packet can be identified as a particle
87
Active Figure 40.19
Use the active figure to
choose the number of
waves to add together
Observe the resulting
wave packet
The wave packet
represents a particle
PLAY
ACTIVE FIGURE
88
Wave Envelope
The dashed line represents the envelope
function.
This envelope can travel through space with a
different speed than the individual waves
89
Active Figure 40.20
Use the active figure to
observe the movement
of the waves and of the
wave envelope
PLAY
ACTIVE FIGURE
90
Speeds Associated with Wave
Packet
The phase speed of a wave in a wave packet is
given by
This is the rate of advance of a crest on a single wave
The group speed is given by
This is the speed of the wave packet itself
phase
v
k
=
g
d
v
dk
=
91
(40.18)
(40.19)
is angular frequency, k is wavenumber
t t
e
2
,
2
= = k
T
Speeds, cont.
The group speed can also be expressed in
terms of energy and momentum
This indicates that the group speed of the
wave packet is identical to the speed of the
particle that it is modeled to represent
( )
2
1
2
2 2
g
dE d p
v p u
dp dp m m
| |
= = = =
|
\ .
92
(40.22)
Electron Diffraction, Set-Up
93
Electron Diffraction,
Experiment
Parallel beams of mono-energetic electrons
that are incident on a double slit
The slit widths are small compared to the
electron wavelength
An electron detector is positioned far from the
slits at a distance much greater than the slit
separation
94
Electron Diffraction, cont.
If the detector collects
electrons for a long
enough time, a typical
wave interference pattern
is produced
This is distinct evidence
that electrons are
interfering, a wave-like
behavior
The interference pattern
becomes clearer as the
number of electrons
reaching the screen
increases
95
Active Figure 40.22
Use the active figure to
observe the
development of the
interference pattern
Observe the destruction
of the pattern when you
keep track of which slit
an electron goes
through
PLAY
ACTIVE FIGURE
96
Electron Diffraction, Equations
A maximum occurs when
This is the same equation that was used for light
This shows the dual nature of the electron
The electrons are detected as particles at a
localized spot at some instant of time
The probability of arrival at that spot is determined
by finding the intensity of two interfering waves
sin d m =
97
Electron Diffraction Explained
An electron interacts with both slits
simultaneously
If an attempt is made to determine
experimentally which slit the electron goes
through, the act of measuring destroys the
interference pattern
It is impossible to determine which slit the electron
goes through
In effect, the electron goes through both slits
The wave components of the electron are present
at both slits at the same time
98
Werner Heisenberg
1901 1976
German physicist
Developed matrix
mechanics
Many contributions
include:
Uncertainty principle
Recd Nobel Prize in 1932
Prediction of two forms of
molecular hydrogen
Theoretical models of the
nucleus
99
Heisenberg (video)
100
The Uncertainty Principle,
Introduction
In classical mechanics, it is possible, in
principle, to make measurements with
arbitrarily small uncertainty
Quantum theory predicts that it is
fundamentally impossible to make
simultaneous measurements of a particles
position and momentum with infinite accuracy
101
Heisenberg Uncertainty
Principle, Statement
2
x
x p A A >
102
(40.23)
Heisenberg Uncertainty
Principle, Explained
It is physically impossible to measure
simultaneously the exact position and exact
momentum of a particle
The inescapable uncertainties do not arise
from imperfections in practical measuring
instruments
The uncertainties arise from the quantum
structure of matter
103
Heisenberg Uncertainty
Principle, Another Form
Another form of the uncertainty principle can
be expressed in terms of energy and time
This suggests that energy conservation can
appear to be violated by an amount AE as
long as it is only for a short time interval At
2
E t A A >
104
(40.24)
Uncertainty Principle (video)
105
Quiz 40.6
106
A particles location is measured and specified as
being exactly at x = 0, with zero uncertainty in the x
direction. How does that location affect the uncertainty
of its velocity component in the y direction?
a) It does not affect it.
b) It makes it infinite.
c) It makes it zero.
Example 40.6
Locating an electron
The speed of an electron is measured to be
5.00 10
3
m/s to an accuracy of 0.00300%.
Find the minimum uncertainty in determining
the position of this electron.
107
Example 40.7
The line width of atomic emissions
Atoms have quantized energy levels similar to those of
Plancks oscillators, although the energy levels of an atom
are usually not evenly spaced. When an atom makes a
transition between states, energy is emitted in the form of a
photon. Although an excited atom can radiate at any time
from t = 0 to t = , the average time interval after excitation
during which an atom radiates is called the lifetime t. If t =
1.0 10
-8
s, use the uncertainty principle to compute the
line width f produced by this finite lifetime.
108
109
Uncertainty Principle, final
The Uncertainty Principle cannot be
interpreted as meaning that a measurement
interferes with the system.
The Uncertainty Principle is independent of
the measurement process.
It is based on the wave nature of matter.
110
Quantum Physics Remark
(video)
111
Das könnte Ihnen auch gefallen
- Optical Sources, Detectors, and Systems: Fundamentals and ApplicationsVon EverandOptical Sources, Detectors, and Systems: Fundamentals and ApplicationsNoch keine Bewertungen
- Black Body RadiationDokument33 SeitenBlack Body RadiationSafnas Kariapper100% (1)
- Introduction To Quantum PhysicsDokument69 SeitenIntroduction To Quantum PhysicsEr Ishan SharmaNoch keine Bewertungen
- Introduction To Quantum PhysicsDokument72 SeitenIntroduction To Quantum PhysicsarunsumbriaNoch keine Bewertungen
- Introduction to Quantum Mechanics Lecture NotesDokument100 SeitenIntroduction to Quantum Mechanics Lecture NotesjeremieNoch keine Bewertungen
- CH 2 The Particle Properties of WavesDokument21 SeitenCH 2 The Particle Properties of Wavesyohanse mehabawNoch keine Bewertungen
- Laporan Eksperimen Fisika: Radiasi Benda HitamDokument7 SeitenLaporan Eksperimen Fisika: Radiasi Benda HitamidhanurNoch keine Bewertungen
- Introduction To Quantum PhysicsDokument74 SeitenIntroduction To Quantum PhysicsultimuNoch keine Bewertungen
- Chem. 266 Physical Chemistry III: Quantum MechanicsDokument70 SeitenChem. 266 Physical Chemistry III: Quantum MechanicsJohn Edward ZapaterNoch keine Bewertungen
- PlanckDokument15 SeitenPlanckPrincess ZoyaNoch keine Bewertungen
- Fisika KuantumDokument43 SeitenFisika KuantumRizna NofitasariNoch keine Bewertungen
- Introduction To Quantum PhysicsDokument64 SeitenIntroduction To Quantum PhysicsDidik Setyawarno 198810132015041004Noch keine Bewertungen
- Quantum Mechanics Notes-Part 1Dokument15 SeitenQuantum Mechanics Notes-Part 1aman bhatiaNoch keine Bewertungen
- PHY704 Lecture On Duel Behabiour of LightDokument55 SeitenPHY704 Lecture On Duel Behabiour of Lightravinesh rattanNoch keine Bewertungen
- Unit-I-Modern Physics & Quantum MechanicsDokument57 SeitenUnit-I-Modern Physics & Quantum Mechanicsmwita mwitaNoch keine Bewertungen
- Modern PhysicsDokument15 SeitenModern Physicsabdogh93Noch keine Bewertungen
- Wave Properties and Photoelectric EffectDokument9 SeitenWave Properties and Photoelectric EffectAmanuel BazeNoch keine Bewertungen
- 02 IntroQuantumPhysicsDokument8 Seiten02 IntroQuantumPhysicscychan410Noch keine Bewertungen
- Quantum MechanicsDokument46 SeitenQuantum MechanicsAbhilash Nair50% (2)
- Bab1 Modern Physics RevisitedDokument31 SeitenBab1 Modern Physics Revisitednurul najwaNoch keine Bewertungen
- Ch0 IntroductionDokument33 SeitenCh0 IntroductionVishalSharmaNoch keine Bewertungen
- Complete Book Modern PhysicsDokument64 SeitenComplete Book Modern Physicssweta gupta100% (1)
- Planck'smanual v1Dokument12 SeitenPlanck'smanual v1spyzer.x.001Noch keine Bewertungen
- Bab 2 Blackbody, PhotoelectricDokument35 SeitenBab 2 Blackbody, PhotoelectricFakrurazi NajmiNoch keine Bewertungen
- Wave Properties of ParticleDokument35 SeitenWave Properties of ParticleAnnida ChairatunisaNoch keine Bewertungen
- Black Body RadiationDokument33 SeitenBlack Body RadiationsindhsanamNoch keine Bewertungen
- Quantization of Light L28Dokument6 SeitenQuantization of Light L28John JohnsonNoch keine Bewertungen
- AP Physics Chapter 27 Quantum PhysicsDokument78 SeitenAP Physics Chapter 27 Quantum PhysicsMythos EntertainmentNoch keine Bewertungen
- Philippine STEM Module Explains Photoelectric EffectDokument12 SeitenPhilippine STEM Module Explains Photoelectric EffectJp menorNoch keine Bewertungen
- MODERN PHYSICS PRINCIPLESDokument66 SeitenMODERN PHYSICS PRINCIPLESAk JaNoch keine Bewertungen
- Notes - Quantum Theory of Light and Matter WavesDokument34 SeitenNotes - Quantum Theory of Light and Matter Wavesphysics olympiad100% (1)
- Quantum Mechanics Introduction ExplainedDokument86 SeitenQuantum Mechanics Introduction ExplainedGame 1Noch keine Bewertungen
- Blackbody Lab ReportDokument2 SeitenBlackbody Lab Reportkashawna fujiwaraNoch keine Bewertungen
- Electromagnetic Radiation Behaving As ParticlesDokument48 SeitenElectromagnetic Radiation Behaving As ParticlesLeo YipNoch keine Bewertungen
- UofT Black BodyDokument7 SeitenUofT Black BodyShayaan ZakaNoch keine Bewertungen
- Unit 11 Modern PhysicsDokument90 SeitenUnit 11 Modern PhysicsPeril LousNoch keine Bewertungen
- Physics112 - Chapter40Dokument72 SeitenPhysics112 - Chapter40Gurpreet SohalNoch keine Bewertungen
- Modern Physics & Electronics Course Code: PHYS2114 Lecture No.1 Black Body RadiationsDokument22 SeitenModern Physics & Electronics Course Code: PHYS2114 Lecture No.1 Black Body RadiationsThis Is Me.Noch keine Bewertungen
- Wave Mechanics and The SCHR Odinger Equation: 1.1 Historical Foundations of Quantum PhysicsDokument9 SeitenWave Mechanics and The SCHR Odinger Equation: 1.1 Historical Foundations of Quantum PhysicsPhyo ThihaNoch keine Bewertungen
- CH103 - Part 1 Physical Chemistry: y y Introduction To BondingDokument33 SeitenCH103 - Part 1 Physical Chemistry: y y Introduction To BondingNimanyu JoshiNoch keine Bewertungen
- QM BasicsDokument12 SeitenQM Basicsh.anurag248Noch keine Bewertungen
- Black Body RadiationDokument12 SeitenBlack Body RadiationMahesh Lohith K.S100% (4)
- Atoms Truc TDokument9 SeitenAtoms Truc Tpavi32Noch keine Bewertungen
- Photometry PDFDokument42 SeitenPhotometry PDFDeepak SharmaNoch keine Bewertungen
- Schroedinger EssayDokument19 SeitenSchroedinger EssayMark MoodyNoch keine Bewertungen
- Electronic Structure of Atoms - General ChemistryDokument92 SeitenElectronic Structure of Atoms - General ChemistryDuc Anh NguyenNoch keine Bewertungen
- 27-1 Planck Solves The Ultraviolet Catastrophe: Chapter 27 - The Quantum World Page 27 - 2Dokument2 Seiten27-1 Planck Solves The Ultraviolet Catastrophe: Chapter 27 - The Quantum World Page 27 - 2JUAN VICTOR ESTRADA MENESESNoch keine Bewertungen
- RadiationDokument19 SeitenRadiationNajmul Islam ShawonNoch keine Bewertungen
- Max Planck and Black Body RadiationDokument3 SeitenMax Planck and Black Body RadiationMarie Joy EngayNoch keine Bewertungen
- 8-Ch7 (Struktur Atom)Dokument91 Seiten8-Ch7 (Struktur Atom)Mia YukimuraNoch keine Bewertungen
- Quantum Chemistry Lecture NotesDokument19 SeitenQuantum Chemistry Lecture NotesSadashiv ChoureNoch keine Bewertungen
- Unit 1 Quantum Mechanics PDFDokument32 SeitenUnit 1 Quantum Mechanics PDFDeveshNoch keine Bewertungen
- B.Tech First Year: Course Name: Engineering PhysicsDokument45 SeitenB.Tech First Year: Course Name: Engineering PhysicsDhyey DESAIIINoch keine Bewertungen
- Physics (4) PHM 124Dokument13 SeitenPhysics (4) PHM 124Hoda ElsayedNoch keine Bewertungen
- Quantum MechanicsDokument35 SeitenQuantum MechanicsJonNoch keine Bewertungen
- 1523611823MS PHY QM Text 1Dokument14 Seiten1523611823MS PHY QM Text 1NaveenNoch keine Bewertungen
- Development of Quantum Mechanics: Earning BjectivesDokument83 SeitenDevelopment of Quantum Mechanics: Earning Bjectivesshubham tejaniNoch keine Bewertungen
- Photoelectric EffectDokument17 SeitenPhotoelectric Effectvprogamer06Noch keine Bewertungen
- Plancks Constant Version 2016-1Dokument10 SeitenPlancks Constant Version 2016-1MR XNoch keine Bewertungen
- Quantum MechanicsDokument38 SeitenQuantum MechanicsSoumyadeep DasNoch keine Bewertungen
- O&od 3Dokument26 SeitenO&od 3doldolzzNoch keine Bewertungen
- Introductory Optics System: Instruction Manual and Experiment Guide For The PASCO Scientific Model OS-8500Dokument75 SeitenIntroductory Optics System: Instruction Manual and Experiment Guide For The PASCO Scientific Model OS-8500Raymond Roy Ocariza ArantonNoch keine Bewertungen
- Case StudyDokument1 SeiteCase StudydoldolzzNoch keine Bewertungen
- Tutorial 1Dokument3 SeitenTutorial 1doldolzzNoch keine Bewertungen
- LubricationDokument21 SeitenLubricationmojiryhamid100% (1)
- Physics SS2 Term 2 Dec 2022Dokument71 SeitenPhysics SS2 Term 2 Dec 2022TahmidNoch keine Bewertungen
- EXP5procedure PDFDokument2 SeitenEXP5procedure PDFGeneva OrañoNoch keine Bewertungen
- Additives Guide - BASFDokument13 SeitenAdditives Guide - BASFShahzaib KhanNoch keine Bewertungen
- Structural mechanics course detailsDokument54 SeitenStructural mechanics course detailsAlhaz UddinNoch keine Bewertungen
- Astm A668, A668m (2020)Dokument10 SeitenAstm A668, A668m (2020)SK A100% (1)
- An Investigation of Tio2-Znfe2o4 Nanocomposites For Visible LightDokument124 SeitenAn Investigation of Tio2-Znfe2o4 Nanocomposites For Visible LightVăn Đại - BKHN100% (1)
- Establishing ASME B16.5 Blind Flanges Pressure Ratings For Reduced Flange Thickness++++++++++Dokument6 SeitenEstablishing ASME B16.5 Blind Flanges Pressure Ratings For Reduced Flange Thickness++++++++++Ali AlizadehNoch keine Bewertungen
- Ian BAB 4Dokument6 SeitenIan BAB 4NURUL SYUHADA BT ISMAIL HAJARNoch keine Bewertungen
- Hyperzone Presentation SPE 2019Dokument17 SeitenHyperzone Presentation SPE 2019phu nghiaNoch keine Bewertungen
- Environmental ResearchDokument13 SeitenEnvironmental ResearchHerald MatiusNoch keine Bewertungen
- Two Reversible Adiabatic Paths Cannot Intersect Each Other:: Reversible Isotherm A B Reversible AdiabaticsDokument31 SeitenTwo Reversible Adiabatic Paths Cannot Intersect Each Other:: Reversible Isotherm A B Reversible Adiabaticsmohit dadarwalNoch keine Bewertungen
- EE305Dokument19 SeitenEE305api-3853441Noch keine Bewertungen
- 42.6-Electrical Quantities-Cie Igcse Physics Ext-Theory-Qp Core 2Dokument14 Seiten42.6-Electrical Quantities-Cie Igcse Physics Ext-Theory-Qp Core 2LiliNoch keine Bewertungen
- Test Bank For Organic Chemistry Fifth EditionDokument5 SeitenTest Bank For Organic Chemistry Fifth EditionJean Taylor100% (35)
- Material Properties & Metal Forming Processes TestDokument5 SeitenMaterial Properties & Metal Forming Processes TestSaikat BanerjeeNoch keine Bewertungen
- Kema Three Core Power Cables - 1Dokument38 SeitenKema Three Core Power Cables - 1Thinh Tien NguyenNoch keine Bewertungen
- Si Content For GalvanizingDokument7 SeitenSi Content For GalvanizingmritunjayNoch keine Bewertungen
- Abstract Book2018Dokument243 SeitenAbstract Book2018SaravananNoch keine Bewertungen
- Tabel Baja WF LRFDDokument8 SeitenTabel Baja WF LRFDBlhoe KhoethoexNoch keine Bewertungen
- Atomic Layer Deposition for Precise Thin Film GrowthDokument19 SeitenAtomic Layer Deposition for Precise Thin Film GrowthShahid Ali LeghariNoch keine Bewertungen
- CFD Simulation of Dust Particle Removal Efficiency of A Venturi Scrubber in CFXDokument9 SeitenCFD Simulation of Dust Particle Removal Efficiency of A Venturi Scrubber in CFXCristian MartínezNoch keine Bewertungen
- Slurry Transport Using Centrifugal Pumps - InDICEDokument5 SeitenSlurry Transport Using Centrifugal Pumps - InDICECarlos Cortés Ramos20% (5)
- Base Slab Design - CulvertDokument6 SeitenBase Slab Design - CulvertSuresh MahalingamNoch keine Bewertungen
- Dislocations and Strengthening: Chapter OutlineDokument19 SeitenDislocations and Strengthening: Chapter OutlineAshok KumarNoch keine Bewertungen
- Physics Crash ss1 To ss2Dokument6 SeitenPhysics Crash ss1 To ss2RaphaelNoch keine Bewertungen
- Nature of Reservoir Fluids Phase Behaviour of Reservoir FluidsDokument83 SeitenNature of Reservoir Fluids Phase Behaviour of Reservoir FluidsAnonymous qaI31HNoch keine Bewertungen
- The Rise of Conductive Copper Inks Challenges and PerspectiveDokument26 SeitenThe Rise of Conductive Copper Inks Challenges and PerspectiveMicu CristiNoch keine Bewertungen
- Minggu 3 Klasifikasi Massa BatuanDokument23 SeitenMinggu 3 Klasifikasi Massa Batuanarwan_sipilNoch keine Bewertungen
- VISCOTAQR PE Outer Wrap February 2014Dokument2 SeitenVISCOTAQR PE Outer Wrap February 2014Sinan A AzizNoch keine Bewertungen