Beruflich Dokumente
Kultur Dokumente
Buerger's Disease
Hochgeladen von
sweetyjonasOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Buerger's Disease
Hochgeladen von
sweetyjonasCopyright:
Verfügbare Formate
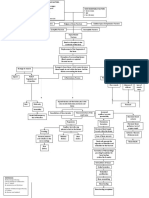
Buergers disease: Smokers beware Buerger's disease, also known as thromboangiitis obliterans, is a rare disease of the small arteries
and veins in the arms and legs. The disease is characterized by narrowing/blockage of the blood vessels, mainly in the hands and feet, leading to blockage of the blood vessel. This eventually leads to gangrene of the fingers or toes. Buerger's disease is rare in the United States, but is very common in the Middle East and Far East. Buerger's disease most commonly affects men between ages 20 and 40, though it's becoming more common in women. With the recent wave of immigrants, the disorder is now becoming more prominent in North America. The majority of individuals diagnosed with buergers disease smoke cigarettes or use chewing tobacco. Quitting all forms of tobacco is the only way to stop Buerger's disease. For those who don't quit, amputation of all or part of a limb may ultimately be necessary. The first reported case of thromboangiitis obliterans was described in Germany in 1879. A little more than a quarter of a century later, in Brooklyn, NY, Leo Buerger published a detailed description of the disease in which he referred to the clinical presentation of thromboangiitis obliterans as "presenile spontaneous gangrene." The paper discussed the pathological findings in 11 limbs amputated from Jewish patients with the disease. It was then discovered that the disorder was intimately associated with smoking and affected the small blood vessels in the hands and the feet. The disorder typically involves the distal vessels with proximal progression and is most often seen in middle aged men between the ages of 20-40, who have a history of smoking. Even though usually seen in men, cases have been reported in women. While more common in Middle and Far Eastern races, the disease is seen universally in all races. While the exact cause of this disorder remains unknown, all patients with TAO are smokers. It is believed that TAO is an autoimmune disorder (your own immune system starts to attack your body), most likely triggered by the use of tobacco. Pathophysiology Buergers disease generally affects the small and medium sized vessels in the body. It may involve the blood vessels in the arms, hands legs and feet. In addition the blood vessels to the kidney, bowels and event the brain may be involved. When the blood vessels are affected, the vessel becomes narrower and eventually closes offresulting in no blood supply. There are numerous reports that the small blood vessels supplying the penis are also blocked and the majority of these individuals also complain of impotence (erectile failure). While the etiology of Buerger disease is unknown, exposure to tobacco is essential for both initiation and progression of the disease. The idea that the condition is linked to tobacco exposure is supported by the fact that the disease is more common in countries with heavy use of tobacco. In the Middle East, Asia and the Pacific where tobacco use is endemic, the condition is extremely common. While the majority of the cases are seen in smokers, a few cases have also been reported in non smokers and this has been attributed to either the use of chewing tobacco or second hand smoke. Pathophysiology Buerger's disease is a vasculitis affecting crural and brachial arteries. Advanced disease may include subclavian and axillary artery involvement. It has been known to affect cerebral, coronary, renal, gonadal, and mesenteric vessels to a much lesser degree. In the acute pathological phase, active inflammation of all 3 vessel layers is seen. In the chronic pathological phase, the thrombus is organised with revascularisation of the medial and adventitial layers. A hypercellular thrombus rich in lymphocytes, fibroblasts, and giant cells fills the vessel lumen. The internal elastic lamina remains intact, with no vessel wall necrosis, calcification, or atheromatous plaques. The vasculitis may be triggered by a hypersensitivity to tobacco constituents. Impaired endothelium-dependent vasodilation has been identified in patients with thromboangiitis obliterans, which may encourage thrombus formation. [14] A cell-mediated immune response to artery collagen components has been suggested. The histocompatibility leukocyte antigen (HLA)-DRA was more frequently found and HLA-DRW6 less frequently found in patients with Buerger's disease compared with smokers without Buerger's disease and non-smokers. [15] Plasma levels of kallikreins and kininase II (componenets of the kinin system of blood proteins) are higher in patients with Buerger's disease who are active smokers compared with non-Buerger's disease patients, whether or not they smoke. These protein levels were significantly greater in patients with Buerger's disease who were active smokers than in those who were ex-smokers. This may indicate that vasodilatation occurs in response to the vascular changes taking place, supporting a theory of immune complex deposition due to nicotine stimulation.[16] PATHOPHYSIOLOGY OF MULTIPLE SCLEROSIS Multiple Sclerosis is classified amongst the autoimmune conditions, which are diseases deriving from a malfunction of the immune system that attacks substances and tissues normally present in the body as it wrongly
recognises them as foreign agents to the body system. In the case of Multiple Sclerosis the immune system attacks the nervous system. MS Lesions The name Multiple Sclerosis refers to lesions (also called plaques or scars) that generate in the nervous system. The majority of MS lesions are located in the white area near the cerebellum, the spinal cord, the brain stem and the optical nerve. When MS lesions are present neurons cannot transmit impulses efficiently. In fact the disease destroys the layer (myelin) which covers the nervous systems fibres and facilitates the neurons transmitting signals to the body. This results in the diminishing or complete disappearance of myelin. A partial restorative process - called remyelination occurs at the early stages of the disease. However, as the cells myelin cover cannot completely be rebuilt; repeated attacks lead to fewer successful remyelinations and thus to the formation of lesions in the irreversibly damaged areas. Inflammation Multiple Sclerosis also generates an inflammatory process. This inflammation is caused by the T-cells which are cells that play a crucial role in the bodys defences. In MS T-cells manage to infiltrate into the brain via the blood-brain barrier, which is both a physical barrier and system of cellular transport. This barrier is not normally accessible to T-cells, unless it is affected by a virus, which reduces the strength of the junctions forming the barrier. T-cells remain then locked inside the brain, wrongly perceiving myelin as an alien agent and attack it as if it were a virus. This generates inflammatory processes and further damaging effects such as swelling and activation of other immune cells and antibodies. PATHOPHYSIOLOGY OF MS Blood-brain barrier breakdown The bloodbrain barrier is a capillary system that should prevent entrance of T cells into the nervous system.[4] The bloodbrain barrier is normally not permeable to these types of cells, unless triggered by infection or a virus, which decreases the integrity of the tight junctionsforming the barrier.[4] When the bloodbrain barrier regains its integrity, usually after infection or virus has cleared, the T cells are trapped inside the brain.[4] Autoimmunology MS is currently believed to be an immune-mediated disorder mediated by a complex interaction of the individual's genetics and as yet unidentified environmental insults.[4]Damage is believed to be caused by the patient's own immune system. The immune system attacks the nervous system, possibly as a result of exposure to a molecule with a similar structure to one of its own.[4] Lesions The name multiple sclerosis refers to the scars (scleroses better known as plaques or lesions) that form in the nervous system. MS lesions most commonly involve white matter areas close to the ventricles of the cerebellum, brain stem, basal ganglia and spinal cord; and the optic nerve. The function of white matter cells is to carry signals between grey matter areas, where the processing is done, and the rest of the body. The peripheral nervous system is rarely involved.[4] More specifically, MS destroys oligodendrocytes, the cells responsible for creating and maintaining a fatty layer known as the myelinsheathwhich helps the neurons carry electrical signals.[4] MS results in a thinning or complete loss of myelin and, as the disease advances, the cutting (transection) of the neuron's extensions or axons. When the myelin is lost, a neuron can no longer effectively conduct electrical signals.[4] A repair process, called remyelination, takes place in early phases of the disease, but the oligodendrocytes cannot completely rebuild the cell's myelin sheath.[29] Repeated attacks lead to successively fewer effective remyelinations, until a scar-like plaque is built up around the damaged axons.[29] Different lesion patterns have been described.[30] Inflammation Apart from demyelination, the other pathologic hallmark of the disease is inflammation. According to a strictly immunological explanation of MS, the inflammatory process is caused by T cells, a kind of lymphocyte. Lymphocytes are cells that play an important role in the body's defenses. [4] In MS, T cells gain entry into the brain via the previously described bloodbrain barrier. Evidence from animal models also point to a role of B cells in addition to T cells in development of the disease.[31]
The T cells recognize myelin as foreign and attack it as if it were an invading virus. This triggers inflammatory processes, stimulating other immune cells and soluble factors like cytokines and antibodies. Leaks form in the bloodbrain barrier, which in turn cause a number of other damaging effects such as swelling, activation of macrophages, and more activation of cytokines and other destructive proteins.[4] Pathophysiology Myelin comprises lipids and protein, serving to insulate and aid in nerve fiber conduction. In multiple sclerosis (MS), plaques (scleroses) form on the myelin coating of nerve fibers in the central nervous system (CNS). When myelin is damaged by plaque formation, nerve fiber conduction is reduced or absent. This phenomenon, demyelination, causes a disruption in the nerve signals sent from the brain. Some nerve fibers, or axons, never recover from the effects of demyelination and become impaired, leading to permanent axonal damage. This demyelination and axonal damage can affect multiple systems and result in symptomatic problems. Huntington's Disease Pathophysiology HD is associated with progressive degeneration of neurons in certain regions of the brain and the presence of astrocytes that accumulate due to destruction of nearby neurons (gliosis). These neurodegenerative changes primarily occur within the caudate nuclei and the putamen, substructures of the basal ganglia that are collectively known as the striatum. (The basal ganglia consist of specialized nerve cell clusters deep within the brain that organize motor behavior. Major substructures of the basal ganglia include the caudate nuclei, the putamen, and the globus pallidus as well as other cell groups.) HD is also characterized by associated neuronal degeneration within the temporal and frontal lobes of the cerebral cortex. This part of the brain is responsible for integrating higher mental functioning, movements, and sensations. The degenerative changes in HD primarily affect certain nerve cells of the striatum known as medium-sized "spiny" neurons, which are named for their size and appearance and project into the globus pallidus and substantia nigra. These highly specialized "spiny" neurons secrete gamma-aminobutyric acid (GABA), a neurotransmitter that inhibits the release of neurotransmitters from other nerve cells. One theory suggests that selective loss of these specialized cells results in decreased inhibition (i.e., increased activity) of the thalamus. Therefore the thalamus increases its output to certain regions of the brain's cerebral cortex. This may lead to the disorganized, excessive (hyperkinetic) movement patterns of chorea. Some studies demonstrate reduced uptake of the neurotransmitter dopamine within the striatum, potentially playing a role in causing the choreic movements associated with HD. Several investigations indicate that impaired energy metabolism (mitochondrial dysfunction) may result in excessive or prolonged activation (excitotoxicity) by neurotransmitters, such as glutamate or Nmethyl-D-aspartate (NMDA). This may cause damage to and loss of nerve cells (apoptosis). (For more on apoptosis, see "Mutant huntingtin protein and intracellular abnormalities.") Evidence suggests that the formation of toxic compounds known as oxygen-free radicals may contribute to striatal cell injury. An imbalance between free radical production and elimination results in an increasing accumulation of these toxins in certain tissues. Eventually, this causes damage and impaired functioning of affected cells. Many researchers theorize that free radicals may play some role in the loss of neurons associated with many neurodegenerative diseases. In patients with HD, positron emission tomography (PET) scanning has shown decreased glucose and oxygen metabolism within the caudate nuclei early in the course of the disease. These findings occur in patients with other neurodegenerative diseases associated with chorea. This lends support to the theory that disturbances in the metabolism of certain neurotransmitters and heightened sensitivity of particular neuroreceptors may contribute to the symptoms associated with HD. Pathophysiology The expanded CAG repeat generates an elongated polyglutamine tail on the huntingtin protein, which leads to cleavage and the generation of toxic fragments of this abnormal protein. [10]The polyglutamine composition of the toxic fragments predisposes them to cross-link, forming aggregates that resist degradation and interfere with a variety of normal cellular functions, particularly mitochondrial energy metabolism. [11] [12] However, these aggregates also interfere with the regulation of transcription, axonal and vesicular transport, apoptosis, proteasome function, and cell-cell interactions. [1] Haploinsufficiency, the reduction in levels of wild-type huntingtin, does not cause disease. [1] However, it may contribute to the adverse effects of aggregates. Therapeutic interventions designed to improve mitochondrial function, block huntingtin cleavage at sites that generate toxic fragments, prevent expression of mutant huntingtin, improve cell-cell interactions, enhance autophagic consumption of mutant proteins, and retard apoptosis are under active investigation. [13] [14] [15]
Huntington's disease primarily affects the striatum, and most clinical features are directly attributable to damage in this area, including cognitive impairment, behavioural changes, and loss of coordination. [4] [16] However, pathological changes occur in multiple cortical and sub-cortical structures as well. [17] Chorea, the most striking feature of Huntington's disease, results from striatal dysfunction.
Das könnte Ihnen auch gefallen
- Metabolic Alkalosis, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsVon EverandMetabolic Alkalosis, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsNoch keine Bewertungen
- Buerger DiseaseDokument3 SeitenBuerger DiseaseElmer DizonNoch keine Bewertungen
- Ebstein Anomaly, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsVon EverandEbstein Anomaly, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsNoch keine Bewertungen
- Case AnalysisDokument12 SeitenCase AnalysisFroilan TaracatacNoch keine Bewertungen
- Avascular Necrosis, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsVon EverandAvascular Necrosis, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsBewertung: 4 von 5 Sternen4/5 (2)
- Clinical PaperDokument18 SeitenClinical PaperlhhjklllNoch keine Bewertungen
- Clavicle FractureDokument4 SeitenClavicle FracturesridharNoch keine Bewertungen
- Hirschsprung’s Disease, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsVon EverandHirschsprung’s Disease, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsNoch keine Bewertungen
- Intracerebral HemorrageDokument13 SeitenIntracerebral HemorrageChristian JuarezNoch keine Bewertungen
- ChickenpoxDokument16 SeitenChickenpoxJeet ThuraiNoch keine Bewertungen
- Pathophysiology of Arteriosclerosis and AtherosclerosisDokument22 SeitenPathophysiology of Arteriosclerosis and Atherosclerosisjoyrena ochondraNoch keine Bewertungen
- Predisposing Conditions, Management and Prevention of Chronic Kidney DiseaseDokument52 SeitenPredisposing Conditions, Management and Prevention of Chronic Kidney DiseaseSaad MotawéaNoch keine Bewertungen
- Tuberculous MeningitisDokument23 SeitenTuberculous MeningitisAsma KhmNoch keine Bewertungen
- DrowningDokument7 SeitenDrowningmedhatsabriNoch keine Bewertungen
- Teaching Case Presentation 2Dokument20 SeitenTeaching Case Presentation 2api-347034408Noch keine Bewertungen
- Appendicitis: Causes, Symptoms, Diagnosis and TreatmentDokument35 SeitenAppendicitis: Causes, Symptoms, Diagnosis and TreatmentleighjagNoch keine Bewertungen
- Nursing Care Plan for Pain ManagementDokument1 SeiteNursing Care Plan for Pain ManagementCharina AubreyNoch keine Bewertungen
- Managing Acute Myocardial Infarction and ICU CareDokument45 SeitenManaging Acute Myocardial Infarction and ICU CareWilmaBongotanPadawilNoch keine Bewertungen
- Brain TumorDokument7 SeitenBrain TumorPintu Kumar100% (1)
- NCP - Self-Concept RT Body Image (Pott's Dse)Dokument3 SeitenNCP - Self-Concept RT Body Image (Pott's Dse)yanny03Noch keine Bewertungen
- Case Study PneumoniaDokument6 SeitenCase Study PneumoniaBrian CornelNoch keine Bewertungen
- Week 14 - CD Course Task 8Dokument1 SeiteWeek 14 - CD Course Task 8Rochelle TenederoNoch keine Bewertungen
- Nursing care of Clients in Emergency Situations - 1Dokument48 SeitenNursing care of Clients in Emergency Situations - 1Danica FrancoNoch keine Bewertungen
- Hepatitis overview and nursing careDokument18 SeitenHepatitis overview and nursing careAnne B. BuenvenidaNoch keine Bewertungen
- Glasgow Coma ScaleDokument3 SeitenGlasgow Coma Scaletoto11885Noch keine Bewertungen
- Au Di Minor Case Study Myasthenia GravisDokument17 SeitenAu Di Minor Case Study Myasthenia Gravisapi-301816885Noch keine Bewertungen
- NCP Impaired SocialDokument5 SeitenNCP Impaired SocialPau-pau BasiNoch keine Bewertungen
- Addison's Disease: Adrenal Insufficiency and Adrenal CrisisDokument15 SeitenAddison's Disease: Adrenal Insufficiency and Adrenal CrisisMaryONoch keine Bewertungen
- Pathophysiology of ArrhythmiasDokument15 SeitenPathophysiology of ArrhythmiasJonathan MontecilloNoch keine Bewertungen
- Closed Humerus Fracture With Radial PalsyDokument35 SeitenClosed Humerus Fracture With Radial PalsyEryn Farahin ZainalNoch keine Bewertungen
- Schematic Diagram BA HAP HRDokument2 SeitenSchematic Diagram BA HAP HRMika MinsalanNoch keine Bewertungen
- I Can'T Breathe If Breathing Is Without: Bronchial AsthmaDokument33 SeitenI Can'T Breathe If Breathing Is Without: Bronchial AsthmaklamorenaNoch keine Bewertungen
- OtosclerosisDokument36 SeitenOtosclerosisShamsheer ShaikNoch keine Bewertungen
- Psych - Chapter 23 Into To Milieu ManagementDokument4 SeitenPsych - Chapter 23 Into To Milieu ManagementKaren かれんNoch keine Bewertungen
- Catheterization & CystoclysisDokument6 SeitenCatheterization & Cystoclysisclariceportin12Noch keine Bewertungen
- Buerger's DiseaseDokument13 SeitenBuerger's DiseaseJoan JuradoNoch keine Bewertungen
- Patho FractureDokument2 SeitenPatho FracturejaninenicoleNoch keine Bewertungen
- HPV Virus Causes Recurrent Respiratory Papillomatosis in BoyDokument21 SeitenHPV Virus Causes Recurrent Respiratory Papillomatosis in BoyDaffa IbnurasyNoch keine Bewertungen
- Pneumonia PresentationDokument20 SeitenPneumonia PresentationsetanpikulanNoch keine Bewertungen
- Dengue Fever: Causes, Symptoms and PreventionDokument7 SeitenDengue Fever: Causes, Symptoms and PreventionAmber Hope PonsicaNoch keine Bewertungen
- Annotated Group 2 Impetigo Concept Mapping 1Dokument30 SeitenAnnotated Group 2 Impetigo Concept Mapping 1DHANE ANN CAMPOSANONoch keine Bewertungen
- Tagum Doctors College, Inc.: Mahogany ST., Rabesubd., Tagum City E-MailDokument4 SeitenTagum Doctors College, Inc.: Mahogany ST., Rabesubd., Tagum City E-MailRoel John Atamosa CasilacNoch keine Bewertungen
- Broncho Pneumonia CSDokument27 SeitenBroncho Pneumonia CSKylie GolindangNoch keine Bewertungen
- Grand Case Presentation Repaired)Dokument45 SeitenGrand Case Presentation Repaired)hulaanmuakoNoch keine Bewertungen
- Pathophysiology of Angina PectorisDokument2 SeitenPathophysiology of Angina PectorisLeroy LoyNoch keine Bewertungen
- Septic ArthritisDokument8 SeitenSeptic ArthritisLorebell100% (2)
- Pleural Fluid Analysis: How The Test Is PerformedDokument4 SeitenPleural Fluid Analysis: How The Test Is PerformedKevin LlorenteNoch keine Bewertungen
- Cavite State University: I. ObjectivesDokument7 SeitenCavite State University: I. ObjectivesChamy CruzNoch keine Bewertungen
- Al-Zaytoonah University ER Nursing LogDokument5 SeitenAl-Zaytoonah University ER Nursing LogTahani KhalilNoch keine Bewertungen
- First Aide 2023Dokument97 SeitenFirst Aide 2023jrfdcafprescomNoch keine Bewertungen
- Cardiac MyxomaDokument4 SeitenCardiac MyxomaAve FenixNoch keine Bewertungen
- History and Assessment & Anatomy and Physiology of BurnsDokument10 SeitenHistory and Assessment & Anatomy and Physiology of BurnsRina MaeNoch keine Bewertungen
- Potts DiseaseDokument8 SeitenPotts Diseaseaimeeros0% (2)
- PneumoniaDokument4 SeitenPneumoniaroscelle100% (1)
- Prioritization of Problems Health Problem:: Carlatan, San Fernando City, La UnionDokument4 SeitenPrioritization of Problems Health Problem:: Carlatan, San Fernando City, La UnionGabrielle John HernaezNoch keine Bewertungen
- Animal and Insect Bites DiseasesDokument28 SeitenAnimal and Insect Bites DiseasesAditya PrabawaNoch keine Bewertungen
- Acute AppendicitisDokument23 SeitenAcute AppendicitisSandie Daniel GabalunosNoch keine Bewertungen
- Sudden Infant Death SyndromeDokument5 SeitenSudden Infant Death SyndromeJanelle Gift SenarloNoch keine Bewertungen
- Pathophysiology of Appendicitis (RupturedDokument2 SeitenPathophysiology of Appendicitis (RuptureddesireeroseNoch keine Bewertungen
- Hyperaldosteronism or Conn's DiseaseDokument3 SeitenHyperaldosteronism or Conn's DiseasesweetyjonasNoch keine Bewertungen
- Garantisadong PambataDokument6 SeitenGarantisadong PambatasweetyjonasNoch keine Bewertungen
- BranchesDokument3 SeitenBranchessweetyjonasNoch keine Bewertungen
- Doctors Order Admission to Crisis Intervention UnitDokument2 SeitenDoctors Order Admission to Crisis Intervention UnitsweetyjonasNoch keine Bewertungen
- DOH Annual Calendar 2013Dokument6 SeitenDOH Annual Calendar 2013Jayson ZubiagaNoch keine Bewertungen
- Normal CBC and Urinalysis Reference RangesDokument12 SeitenNormal CBC and Urinalysis Reference RangessweetyjonasNoch keine Bewertungen
- DianneDokument1 SeiteDiannesweetyjonasNoch keine Bewertungen
- What Is Diabetes MellitusDokument1 SeiteWhat Is Diabetes MellitusBe NjNoch keine Bewertungen
- Cerebrovascular Accident Written Report For ElectiveDokument9 SeitenCerebrovascular Accident Written Report For ElectivesweetyjonasNoch keine Bewertungen
- Stroke PArt 2Dokument6 SeitenStroke PArt 2sweetyjonasNoch keine Bewertungen
- Colon CancerDokument2 SeitenColon CancersweetyjonasNoch keine Bewertungen
- g4 - Stress Analysis of Operating Gas Pipeline Installed by HorizontalDokument144 Seiteng4 - Stress Analysis of Operating Gas Pipeline Installed by HorizontalDevin DickenNoch keine Bewertungen
- Swatchh Bharat AbhiyanDokument13 SeitenSwatchh Bharat AbhiyanHRISHI SHARMANoch keine Bewertungen
- Guidance Notes Blow Out PreventerDokument6 SeitenGuidance Notes Blow Out PreventerasadqhseNoch keine Bewertungen
- 12 Week Heavy Slow Resistance Progression For Patellar TendinopathyDokument4 Seiten12 Week Heavy Slow Resistance Progression For Patellar TendinopathyHenrique Luís de CarvalhoNoch keine Bewertungen
- Sri Radhakrishna SwamijiDokument43 SeitenSri Radhakrishna SwamijiNarayana IyengarNoch keine Bewertungen
- Smart Grid Standards GuideDokument11 SeitenSmart Grid Standards GuideKeyboardMan19600% (1)
- Plate-Load TestDokument20 SeitenPlate-Load TestSalman LakhoNoch keine Bewertungen
- Diia Specification: Dali Part 252 - Energy ReportingDokument15 SeitenDiia Specification: Dali Part 252 - Energy Reportingtufta tuftaNoch keine Bewertungen
- Idioms & Phrases Till CGL T1 2016Dokument25 SeitenIdioms & Phrases Till CGL T1 2016mannar.mani.2000100% (1)
- Canon imageFORMULA DR-X10CDokument208 SeitenCanon imageFORMULA DR-X10CYury KobzarNoch keine Bewertungen
- WL 318 PDFDokument199 SeitenWL 318 PDFBeckty Ahmad100% (1)
- 1989 GMC Light Duty Truck Fuel and Emissions Including Driveability PDFDokument274 Seiten1989 GMC Light Duty Truck Fuel and Emissions Including Driveability PDFRobert Klitzing100% (1)
- Aircraft Design Project 2Dokument80 SeitenAircraft Design Project 2Technology Informer90% (21)
- Fraktur Dentoalevolar (Yayun)Dokument22 SeitenFraktur Dentoalevolar (Yayun)Gea RahmatNoch keine Bewertungen
- VT6050 VT6010 QuickGuide ENDokument19 SeitenVT6050 VT6010 QuickGuide ENPriyank KumarNoch keine Bewertungen
- Essentials For Professionals: Road Surveys Using SmartphonesDokument25 SeitenEssentials For Professionals: Road Surveys Using SmartphonesDoly ManurungNoch keine Bewertungen
- TutorialDokument324 SeitenTutorialLuisAguilarNoch keine Bewertungen
- Ro-Buh-Qpl: Express WorldwideDokument3 SeitenRo-Buh-Qpl: Express WorldwideverschelderNoch keine Bewertungen
- Clausius TheoremDokument3 SeitenClausius TheoremNitish KumarNoch keine Bewertungen
- European GMP Annex 1 - 2008 Edition - 'Pmeasuring'Dokument3 SeitenEuropean GMP Annex 1 - 2008 Edition - 'Pmeasuring'Khairul AnwarNoch keine Bewertungen
- Juan Martin Garcia System Dynamics ExercisesDokument294 SeitenJuan Martin Garcia System Dynamics ExercisesxumucleNoch keine Bewertungen
- Helmitin R 14030Dokument3 SeitenHelmitin R 14030katie.snapeNoch keine Bewertungen
- WOOD Investor Presentation 3Q21Dokument65 SeitenWOOD Investor Presentation 3Q21Koko HadiwanaNoch keine Bewertungen
- Gotham City: A Study into the Darkness Reveals Dangers WithinDokument13 SeitenGotham City: A Study into the Darkness Reveals Dangers WithinajNoch keine Bewertungen
- QP (2016) 2Dokument1 SeiteQP (2016) 2pedro carrapicoNoch keine Bewertungen
- Fundermax Exterior Technic 2011gb WebDokument88 SeitenFundermax Exterior Technic 2011gb WebarchpavlovicNoch keine Bewertungen
- Laser Surface Treatment ProcessesDokument63 SeitenLaser Surface Treatment ProcessesDIPAK VINAYAK SHIRBHATENoch keine Bewertungen
- Hypophosphatemic Rickets: Etiology, Clinical Features and TreatmentDokument6 SeitenHypophosphatemic Rickets: Etiology, Clinical Features and TreatmentDeysi Blanco CohuoNoch keine Bewertungen
- Convocation ProgramDokument125 SeitenConvocation ProgramZirak TayebNoch keine Bewertungen
- Background of The Study Statement of ObjectivesDokument4 SeitenBackground of The Study Statement of ObjectivesEudelyn MelchorNoch keine Bewertungen
- LIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionVon EverandLIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionBewertung: 4 von 5 Sternen4/5 (402)
- Why We Die: The New Science of Aging and the Quest for ImmortalityVon EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityBewertung: 3.5 von 5 Sternen3.5/5 (2)
- Outlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisVon EverandOutlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisBewertung: 4 von 5 Sternen4/5 (1)
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedVon EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedBewertung: 5 von 5 Sternen5/5 (78)
- The Age of Magical Overthinking: Notes on Modern IrrationalityVon EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityBewertung: 4 von 5 Sternen4/5 (13)
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeVon EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeNoch keine Bewertungen
- The Comfort of Crows: A Backyard YearVon EverandThe Comfort of Crows: A Backyard YearBewertung: 4.5 von 5 Sternen4.5/5 (23)
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsVon EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsBewertung: 3.5 von 5 Sternen3.5/5 (3)
- Techniques Exercises And Tricks For Memory ImprovementVon EverandTechniques Exercises And Tricks For Memory ImprovementBewertung: 4.5 von 5 Sternen4.5/5 (40)
- Raising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsVon EverandRaising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsBewertung: 5 von 5 Sternen5/5 (1)
- Raising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsVon EverandRaising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsBewertung: 4.5 von 5 Sternen4.5/5 (169)
- The Obesity Code: Unlocking the Secrets of Weight LossVon EverandThe Obesity Code: Unlocking the Secrets of Weight LossBewertung: 5 von 5 Sternen5/5 (4)
- The Ultimate Guide To Memory Improvement TechniquesVon EverandThe Ultimate Guide To Memory Improvement TechniquesBewertung: 5 von 5 Sternen5/5 (34)
- Roxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingVon EverandRoxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingBewertung: 5 von 5 Sternen5/5 (4)
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaVon EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- By the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsVon EverandBy the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsNoch keine Bewertungen
- Summary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisVon EverandSummary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisBewertung: 5 von 5 Sternen5/5 (8)
- Roxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingVon EverandRoxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingBewertung: 3.5 von 5 Sternen3.5/5 (33)
- Dark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Von EverandDark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Bewertung: 4.5 von 5 Sternen4.5/5 (110)
- Summary: It Didn't Start with You: How Inherited Family Trauma Shapes Who We Are and How to End the Cycle By Mark Wolynn: Key Takeaways, Summary & AnalysisVon EverandSummary: It Didn't Start with You: How Inherited Family Trauma Shapes Who We Are and How to End the Cycle By Mark Wolynn: Key Takeaways, Summary & AnalysisBewertung: 5 von 5 Sternen5/5 (3)
- The Happiness Trap: How to Stop Struggling and Start LivingVon EverandThe Happiness Trap: How to Stop Struggling and Start LivingBewertung: 4 von 5 Sternen4/5 (1)
- The Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeVon EverandThe Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeBewertung: 4.5 von 5 Sternen4.5/5 (253)
- The Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsVon EverandThe Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsNoch keine Bewertungen
- The Tennis Partner: A Doctor's Story of Friendship and LossVon EverandThe Tennis Partner: A Doctor's Story of Friendship and LossBewertung: 4.5 von 5 Sternen4.5/5 (4)