Beruflich Dokumente
Kultur Dokumente
Analysis Organic Acids HPLC
Hochgeladen von
Gillian WongOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Analysis Organic Acids HPLC
Hochgeladen von
Gillian WongCopyright:
Verfügbare Formate
Journal of Chromatography A, 977 (2002) 8996 www.elsevier.
com / locate / chroma
Separation and determination of organic acids and phenolic compounds in fruit juices and drinks by high-performance liquid chromatography
Guanghou Shui, Lai Peng Leong*
Food Science and Technology Programme, Department of Chemistry, National University of Singapore, S3 -06, Science Drive 4, Singapore 117543, Singapore Received 9 July 2002; received in revised form 22 August 2002; accepted 22 August 2002
Abstract A high-performance liquid chromatographic (HPLC) separation method with photo-diode array detection has been developed for the simultaneous determination of organic acids and phenolic compounds in juices and drinks. The chromatographic analysis of organic acids and phenolic compounds was carried out after their elution with sulphuric acid solution (pH 2.5) and methanol from C 18 stationary phase. The mobile phase employed was sulphuric acid solution working at a ow-rate of 0.35 ml min 21 for the whole run, while methanol was linearly increased to 0.45 ml min 21 from 15 to 75 min followed by a 5-min isocratic elution. Ten organic acid acids were eluted in 30 min and 21 phenolic compounds, which include phenolic acids and avonoids, were eluted in the following 50 min. Target compounds were detected at 215 nm. The repeatability (n53) and between day precision of peak area (n53) were all within 5.0% RSD. The within-day repeatability (n53) and between-day precision (n510) of retention times were within 0.3 and 1.6% relative standard deviation (RSD), respectively. The accuracy of the method was conrmed with an average recovery ranging between 85 and 106%. The method was successfully used to measure a variety of organic acids and phenolic compounds in juices and beverages. This method could also be used to evaluate the authenticity, spoilage or micronutrient contents of juices. 2002 Elsevier Science B.V. All rights reserved. Keywords: Fruit juices; Food analysis; Organic acids; Phenolic compounds
1. Introduction Organic acids are widely distributed in fruits and vegetables. They are also used extensively as food acidulants in the manufacturing of beverages, fruit and vegetable drinks or juices. The principal acids used to enhance beverage avours are citric, tartaric, fumaric and phosphoric acids. Citric acid is the most
*Corresponding author. Fax: 165-6775-7895. E-mail address: laipeng@nus.edu.sg (L.P. Leong).
widely used acid while malic and tartaric acid are important natural compounds of fruits that are used along with fumaric acid in fruit-avoured drinks. In addition, benzoic acid is widely used as a preservative in fruit drinks and juices because the pH imparted by natural and added acids is not sufcient to ensure long-term microbial stability. The content of organic acids in fruit juices not only inuences their avour but also their stability, nutrition, acceptability and keeping quality. Therefore, it is important to be able to precisely determine food acids
0021-9673 / 02 / $ see front matter 2002 Elsevier Science B.V. All rights reserved. PII: S0021-9673( 02 )01345-6
90
G. Shui, L.P. Leong / J. Chromatogr. A 977 (2002) 8996
present for quality control purposes, as well as for meeting various laws and regulations and for labelling purposes. Phenolic compounds widely exist in fruits and vegetables and are reported to have multiple biological effects, including antioxidant activity [1], antitumor [2], antimutagenic [3], and antibacterial and angioprotective properties [4]. Lately, more interest has been shown in these compounds due to their possible additional health benets. This group of compounds mainly include hydroxybenzoic acids, hydroxycinnamic acids, avonoids and isoavonoids. The determination of some phenolic acids using chromatographic methods include gas chromatography (GC) [5,6], capillary electrophoresis (CE) [7] and HPLC [812]. For the analysis of avonoids in foods by HPLC, as reviewed by Merken and Beecher [13], columns are almost exclusively reversed-phase, and elution systems are usually binary, with an aqueous acidied polar solvent and a less polar organic solvent. The aqueous acidied polar solvent included acetic acid, phosphoric acid, perchloric acid, or formic acid. The elution may be isocratic or gradient. Further chromatographic separation and quantication of phenolic acids and avonoids in natural fruits and beverages has been reported by Zuo and coworkers [14,15]. In most of the previously developed methods, signicant restrictions are placed on the compounds to be analysed, either for carboxylic acids or one or two subclasses of phenolic compounds. In addition, chemical derivatizations and solid-phase extraction (SPE) are usually involved in the assay of these target compounds. The main difculty arises from low resolution among the organic acids and phenolic compounds. In addition, large differences in the levels of phenolic compounds in a juice or beverage usually complicate the simultaneous analysis of different classes of phenolic compounds. The purpose of this study is to separate, identify and quantify common organic acids and phenolic compounds in a variety of juices and beverages using HPLC with photodiode array detection (DAD), which will identify compounds not only by their retention times but also their individual spectra. This will not only provide more nutritional data for a juice or beverage but also an indicator of microbial spoilage such as an increase in lactic acid
content indicating lactic acid bacteria spoilage [7] and the authenticity of the juices.
2. Experimental
2.1. Chemicals and standards
L-Ascorbic acid, butylated hydroxytoluene (BHT), (2)-quinic acid, malonic acid, lactic acid, fumaric acid, p-coumaric acid, vanillic acid, myricertin, quercetin, (2)-epicatechin, (2)-gallocatechin, (2)epicatechin gallate, (2)-epigallocatechin, (2)-epigallocatechin gallate (EGCG), (2)-catechin gallate, kaempferol, and eugenol were purchased from Sigma (St Louis, MO, USA); (1)-catechin hydrate, ferulic acid, chlorogenic acid from Aldrich (Milwaukee, WI, USA); trans-cinnamic acid, salicyclic acid, syringic acid, caffeic acid, gallic acid from Acros Organics (Fairlawn, NJ, USA); ethanol, acetic acid, benzoic acid, malic acid, oxalic acid, L-(1)-tartaric acid, hydrochloric acid and HPLC-grade methanol were purchased from Merck (Darmstadt, Germany); citric acid and sulfuric acid from BDH (Poole, UK). The individual standards were dissolved in 0.2% BHT methanol or pH 2.5 sulfuric acid and injected to determine individual retention times. Stock solutions of 500 mg / ml of tartaric acid, 2000 mg / ml quinic acid, 150 mg / ml oxalic acid, 1000 mg / ml malic acid, 100 mg / ml L-ascorbic acid, 1000 mg / ml malonic acid, 1800 mg / ml citric acid, 2000 mg / ml acetic acid, 1000 mg / ml citric acid, and 20 mg / ml fumaric acid were prepared by dissolving pure standards into pH 2.5 sulphuric acid. The stock solutions and the four diluted standards up to 10 times dilution from stock solutions were injected for linearity range and detection limit tests. Similarly, stock solutions of other individual standards were prepared by dissolving 2 mg pure standard in 0.2% BHT methanol. The stock solutions and the four diluted standards up to 20 times dilution from stock solutions were injected for linearity range and detection limit tests. (2)-Gallocatechin, (2)-epigallocatechin, (1)catechin, chlorogenic acid, (2)-epigallocatechin gallate, (2)-epicatechin, caffeic acid, (2)-catechin gallate, ellagic acid, myricetin and quercetin, eugenol and kaempferol were dissolved in about 30 ml of
G. Shui, L.P. Leong / J. Chromatogr. A 977 (2002) 8996
91
0.2% BHT methanol. This solution was mixed with 30 ml of sonicated water. As methanol will interfere with peak shape (see Section 3.3.3), the mixed solution was further evaporated to remove most of the methanol at room temperature under vacuum. Other compounds were dissolved in 50 ml of H 2 SO 4 solution (pH 2.5), and mixed with the above solution. The nal volume of this solution was made up to 100 ml. The solution was kept at 4 8C and used to optimize the HPLC separation conditions. The solution was also applied to observe the within-day and between-day precisions of retention times and peak areas. All solutions were ltered through a 0.45-mm membrane lter (Iwaki Glass) before HPLC analysis, and the mobile phase solvents were degassed before use.
brane lter (Iwaki Glass) and injected directly into the HPLC.
2.5. Chromatographic conditions
Optimum efciency of separation was obtained using 0.35 ml / min of pH 2.5 sulfuric acid solution (solvent A), and the ow-rate of methanol (solvent B) was increased from 0 to 0.45 ml / min from 15 to 75 min and kept at 0.45 min / l for a period of 5 min and then reduced to initial conditions in another 5 min. Ten minutes of equilibration is required before the next injection. Other parameters adopted were as follows: injection volume, 20 ml; column temperature, 40 8C; detection wavelength, 215 nm and UV spectra, 200600 nm range.
2.2. Samples
Fruit juices and drinks were purchased from convenient stores and local supermarkets. Brand A apple juices (Australia) are 100% fresh apple juice without addition of preservative and sugar. Percentage of juice in Brand B apple juice drink (Malaysia) is unknown. Brand C juice drink (USA) contains 18% fruit juice, which includes raspberry, cranberry, apple and grape juice.
3. Results and discussion
3.1. Method development
After multiple preliminary assays, an elution program using methanolH 2 SO 4 water as solvent was chosen. This program allowed carboxylic acids and phosphoric acid to be eluted in 30 min and then phenolic compounds. Fig. 1 illustrates the separation of a standard mixture of 29 acids and phenolic compounds. As shown in Fig. 1, a good separation can be achieved in 80 min. It is essential to keep the ow-rate of solvent A at 0.35 ml / min during the whole run and increase the ow-rate of solvent B to 0.45 ml / min from 15 to 75 min to achieve good resolution of the target compounds since both solvent polarity and pH are very important for separation of target compounds. Table 1 lists the retention times of individual carboxylic acids and phenolic compounds. As can be seen from the chromatogram and Table 1, most target compounds are separated well. However, some compounds such as (2)-epicatechin and p-coumaric acid may be eluted with similar retention times, and thus it is difcult to identify and quantify them by simply using retention time and spiking test. In this experiment, HPLCDAD was used to obtain the spectrum of individual compounds, which could be used to further conrm the unknown peaks in samples.
2.3. Apparatus
The HPLC analyses were carried out using a Shimadzu class LC-VP HPLC system with class LC-VP software, a pump (LC-10ATvp), an autosampler (SIL-10ADvp), and a diode-array detector (SPD-M10Avp) (Shimadzu, Kyoto, Japan). The separation was carried out on a Shim-Pack VP-ODS column (25034.6 mm I.D.) (Shimadzu, Kyoto, Japan) with a guard column (GVP-ODS, 1034.6 mm I.D.).
2.4. Sample preparation
Direct analysis. Juices and beverages were kept at 4 8C before analysis. Fruit juice was centrifuged at 5000 rpm for 10 min. The supernatant was ltered through a 0.45-mm membrane lter before injection. Fruit drinks were ltered through a 0.45-mm mem-
92
G. Shui, L.P. Leong / J. Chromatogr. A 977 (2002) 8996
Fig. 1. HPLC of the mixture of standards. Detection at 215 nm. 1, Tartaric acid; 2, oxalic acid; 3, malic acid; 4, L-ascorbic acid; 5, malonic acid; 6, lactic acid; 7, acetic acid; 8, citric acid; 9, fumaric acid; 10, gallic acid; 11, (2)-catechin gallate; 12, (2)-epigallocatechin; 13, (1)-catechin; 14, p-hydroxybenzoic acid; 15, chlorogenic acid; 16, (2)-EGCG; 17, (2)-epicatechin; 18, caffeic acid; 19, syringic acid; 20, (2)-catechin gallate; 21, ferulic acid; 22, benzoic acid; 23, ellagic acid; 24, salicyclic acid; 25, myricetin; 26, trans-cinnamic acid; 27, quercetin; 28, eugenol; 29, kaempferol.
3.2. Method validation
The within-day repeatability (n53) and betweenday precision (n510) of retention times were within 0.3 and 1.6% relative standard deviation (RSD), respectively. The repeatability (n53) and between day precision (n53) of peak area except L-ascorbic acid, which was not very stable, were all within 5.0% RSD. The accuracy of the method was conrmed by analyzing the mixture prepared by adding suitable amounts of standard mixture to juices with known contents of these target compounds. The recoveries of target compounds were between 85 and 106%. The limit of detection (LOD, S /N53) of individual compounds at 215 nm is given in Table 1. Compounds, such as L-ascorbic acid, benzoic acid and p-hydroxybenzoic acid, will have lower LOD at their maximum absorbance wavelength than those at 215 nm.
3.3. Applications 3.3.1. Determination of organic acids and phenolic compounds in pure apple juice and apple juice drink Apple juice contains a variety of organic acids and phenolic compounds such as malic acid, ascorbic acid, chlorogenic acid and avonoids. Fig. 2 gives the chromatographic prole of Brand A apple juice. Measurements of organic acids and phenolic compounds are useful for labelling purpose as well as for the determination of the authenticity of the juice. For example, the levels of fumaric acid in apple juice could be important indicators of microbial spoilage of juices such as fumaric acid produced by moulds (signicantly by Rhizopus stolonifer) [16], processing of decayed fruits, or addition of synthetic malic acid, which contains fumaric acid as a minor contaminant [17]. The natural content of fumaric acid in freshly prepared claried apple juices without heat treatment varies from 0 to 1.7 mg / l [18]. During heat
G. Shui, L.P. Leong / J. Chromatogr. A 977 (2002) 8996 Table 1 Linear range and limit of detection of carboxylic acid and phenolic compounds Common name Tartaric acid (2)-Quinic acid Oxalic acid DL-Malic acid L-Ascorbic acid Malonic acid Lactic acid Acetic acid Citric acid Fumaric acid Gallic acid (2)-Gallocatechin (2)-Epigallocatechin (1)-Catechin p-Hydroxybenzoic acid Chlorogenic acid (2)-EGCG (2)-Epicatechin p-Coumaric acid Caffeic acid Syringic acid (2)-Catechin gallate Ferulic acid Benzoic acid Ellagic acid Salicyclic acid Myricetin trans-Cinnamic acid Quercetin Eugenol Kaempferol Retention time, t R (min) 11.24 11.37 11.92 13.58 14.58 15.30 16.26 17.06 22.88 25.61 35.28 39.32 43.73 44.47 46.25 46.61 47.70 48.56 48.79 49.15 50.20 53.76 55.51 59.61 62.04 63.50 64.05 69.57 71.39 76.67 78.26 Concentration range (mg / l) 50500 2002000 15150 1001000 10100 1001000 1801800 2002000 1001000 220 1.020 1.020 1.020 1.020 1.020 1.020 1.020 1.020 1.020 1.020 1.020 1.020 1.020 1.020 1.020 1.020 1.020 1.020 1.020 1.020 1.020 a 4109 1073 20 333 1964 53 183 2478 1417 1224 2424 263 676 340 231 169 183 117 270 163 184 105 184 79 339 220 713 171 884 189 394 147 866 217 760 198 614 102 981 62 388 44 703 108 380 139 015 130 278 114 287 66 253 90 207 b 48 457 514 10 319 24 849 102 130 263 204 3499 220 151 13 039 64 360 57 743 35 273 219 483 146 362 83 096 88 640 73 747 95 802 43 283 294 063 92 128 2112 171 234 506 47 227 8268 18 898 186 640 27697 91 952 55 598 8556 r2 0.9987 0.9992 0.9999 0.9902 0.9957 0.9988 0.9999 0.9993 0.9993 0.9987 0.9903 0.9999 1.0000 0.9998 0.9972 0.9922 1.0000 0.9977 0.9989 0.9919 0.9991 0.9912 0.9972 0.9997 0.9992 0.9965 0.9960 0.9974 0.9999 0.9999 1.0000
93
LOD (mg / l) 2.20 12.50 0.94 5.17 0.25 6.00 9.64 10.91 5.93 0.12 0.02 0.04 0.06 0.04 0.09 0.12 0.09 0.04 0.04 0.05 0.03 0.05 0.08 0.18 0.29 0.13 0.06 0.06 0.03 0.16 0.15
processing (evaporation, pasteurisation, and sterilisation) of apple juices, the content of fumaric acid increases slightly due to malic acid dehydration. The content of fumaric acid of well-prepared, authentic and fresh apple juice normally does not exceed 3 mg / l [19]. As shown in Fig. 2(A), malic acid and L-ascorbic acid were probably the main organic acids in Brand A apple juice. The content of fumaric acid in Brand A apple juice was found to be 1.1 mg / l, which is within the normal range of freshly prepared apple juice. In addition, the phenolics prole can be used as an indicator of adulteration of apple juice [20]. Fig. 2(B) shows the chromatographic prole of phenolic compounds in Brand A juice. Chlorogenic acid, (2)-
epicatechin, (2)-EGCG and (2)-epigallocatechin were identied in Brand A apple juice. In addition, several other peaks, named unidentied compounds (UC), have similar spectra to phenolic compounds or benzoic acid. UC1 and UC 4 have similar spectra to that of benzoic acid. UC 2 has a similar spectrum to that of (1)-catechin while UC 3, UC 5 and UC 6 have similar spectra to that of cinnamic acid or syringic acid. In addition, UC2, 3, 5 and 6 all have a maximum absorbance wavelength of 280 nm, indicating their possibilities as phenolic compounds. The low content of fumaric acid and a variety of phenolic compounds in Brand A apple juice indicates its authenticity and good quality. Fig. 3 shows chromatogram of Brand B apple juice drink. Fumaric acid content in this apple juice
94
G. Shui, L.P. Leong / J. Chromatogr. A 977 (2002) 8996
of pure apple juice were higher than in the juice drink.
Fig. 2. Chromatogram of Brand A apple juice showing carboxylic acid and phenolic compounds proles: (A) 030 min; (B) 3080 min. 1, Malic acid; 2, L-ascorbic acid; 3, fumaric acid; 4, UC 1; 5, (2)-epigallocatechin; 6, UC 2; 7, chlorogenic acid; 8, (2)-EGCG; 9, (2)-epicatechin; 10, UC 3; 11, UC 4; 12, UC 5; 13, UC 6.
drink was found to be 10 mg / l. This could be due to the addition of synthetic malic acid. Chlorogenic acids, (2)-epicatechin and other phenolic compounds
3.3.2. Determination of organic acids and phenolic compounds in Brand C juice drink and other applications Bottled Brand C juice drink was found to contain quinic acid, malic acid, L-ascorbic acid, citric acid, fumaric acid and gallic acid (Fig. 4). High content of fumaric acid is likely an indication of microbial spoilage due to the presence of Rhizopus stolonifer. In this study, the contents of phenolic compounds were low. No aglycones were identied in Brand C juice drink, this could be due to a low content of pure juice (18%) that includes cranberry, raspberry and apple juice, and avonols in fruit juice mainly existed as glycosides [20]. The present method could also be used directly to analyse organic acids and free phenolic acids and avonoids in fruits, vegetables and other plants. This method was successfully used by the authors to identify organic acid and avonoids in star fruit juice and residue extract, and the result will be reported soon. This method is also being used for analysis of other fruits and vegetables by members of the research group. It should be stated that, as avonoids and phenolic acids usually occur as glycosides or simple esters or are bound to the cell wall, hydrolysis before analysis is required to liberate them from their bound forms. Acid, base or enzyme catalysed hy-
Fig. 3. Chromatogram of Brand B apple juice drink showing carboxylic acids and phenolic proles at 215 nm. 1, Malic acid; 2, L-ascorbic acid; 3, fumaric acid; 4, chlorogenic acid; 5, benzoic acid.
Fig. 4. Chromatogram of Brand C juice drink showing carboxylic acids and phenolic contents at 215 nm. 1, Quinic acid; 2, malic acid; 3, L-ascorbic acid; 4, citric acid; 5, fumaric acid; 6, gallic acid.
G. Shui, L.P. Leong / J. Chromatogr. A 977 (2002) 8996
95
drolysis processes are usually employed as an aid to structural elucidation and characterisation of glycosides [12]. However, when solvent extraction or solvent-involved hydrolysis is used, care should be taken as solvent might inuence the resolution of target compounds. In addition, t R for phosphoric acid is 9.3 min, which indicates that this method could also be applied in the analysis of other non-fruit beverages.
3.3.3. Negative effects on chromatographic proles by sample solvents It was found that when organic solvent such as methanol was used in sample treatment, the resolution of quinic acid, oxalic acid, malic acid, L-ascorbic acid and malonic acid peaks was decreased (Fig. 5). It was found that if methanol is less than 10%, the resolution is still acceptable although
the t R values were slightly reduced. This might be due to close distribution coefcients and the competitive adsorption behaviour of methanol and those components between mobile phase and stationary phase. Ethanol and acetone, two other frequently-used extraction solvents, may reduce the retention time of organic acids or inuence their chromatogram proles before 30 min, but not phenolic compounds. Therefore, it is necessary to remove most of these organic solvents before HPLC assay if the peaks of interest fall within these time frames.
4. Conclusions A simple method was developed for simultaneous determination of organic acids and phenolic compounds in juices and drinks by HPLC with photodiode array UV detector. Ten non-phenolic acids and 21 phenolic compounds were eluted in 80 min. The established method was successfully used to measure a variety of organic acids and phenolic compounds in fruit juices and drinks. This method could also be used to evaluate the authenticity, spoilage or nutrient contents of juices.
References
[1] C.A. Rice-Evans, N.J. Miller, G. Pagana, Free Radic. Biol. Med. 20 (1996) 933. [2] M.T. Huag, R.C. Smart, C.Q. Wong, A.H. Conney, Cancer Rec. 48 (1988) 5941. [3] H. Hayatsu, S. Arimoto, T. Negishi, Mutat. Res. 202 (1990) 1678. [4] B. Vennat, A. Pourrat, H. Pourrat, D. Gross, P. Bastide, J. Bastide, Chem. Pharm. Bull. 36 (1988) 828. [5] T.J. Barden, M.Y. Croft, E.J. Murby, R.J. Wells, J. Chromatogr. A 785 (1997) 251. [6] F. Ahmet Ayaz, A. Kadiolu, M. Reunanen, J. Agric. Food Chem. 45 (1997) 2539. [7] F. Kvasnicka, M. Voldrich, J. Chromatogr. A 891 (2000) 175. [8] B. Fernandez de Simon, J. Perez-Iizarbe, T. Hernandez, C. Gomez-Cordoves, I. Estrella, J. Agric. Food Chem. 40 (1992) 1531. [9] G.A. Spanos, R.E. Wrolstad, J. Agric. Food Chem. 38 (1990) 1565.
Fig. 5. Negative effects on chromatographic prole by methanol. (A) Standards dissolved in methanolwater (25:75, v / v); (B) Standards dissolved in water. 1, Tartaric acid; 2, oxalic acid; 3, malic acid; 4, L-ascorbic acid; 5, malonic acid; 6, lactic acid.
96
G. Shui, L.P. Leong / J. Chromatogr. A 977 (2002) 8996 [15] Y. Zuo, H. Chen, Y. Deng, Talanta 57 (2002) 307. [16] C. Tricard, M.H. Salagoity, P. Sudraud, Ann. Falsif. Expert. Chim. Toxicol. 79 (1986) 303. [17] R.S. Singhal, P.R. Kulkarni, D.V. Rege, Handbook of Indices of Food Quality and Authenticity, Woodhead, Cambridge, 1997. [18] H.S. Lee, R.E. Wrolstad, J. Assoc. Off. Anal. Chem. 71 (1988) 789. [19] C. Jinge, C. Spandinger, Flussiges Obst. 49 (1982) 57. [20] E.D. Coppola, Food Technol. 38 (1984) 88.
[10] S.H. Hakkinen, S.O. Karenlampi, I.M. Heinonen, H.M. Mykkanen, A.R. Torronen, J. Sci. Food Agric. 77 (1998) 543. [11] A. Escarpa, M.C. Gonzalez, J. Chromatogr. A 830 (1999) 301. [12] H. Chen, Y.G. Zuo, Y.W. Deng, J. Chromatogr. A 913 (2001) 387. [13] H.M. Merken, G.R. Beecher, J. Agric. Food Chem. 48 (2000) 577. [14] Y. Zuo, C. Wang, J. Zhan, J. Agric. Food Chem. 50 (2002) 3789.
Das könnte Ihnen auch gefallen
- Diabetes PresentationDokument31 SeitenDiabetes PresentationAlexis DeCiccaNoch keine Bewertungen
- Understanding The Pathophysiology of IBSDokument7 SeitenUnderstanding The Pathophysiology of IBSCandra Dwipayana HamdinNoch keine Bewertungen
- Diabetes Case Study - Jason BaoDokument8 SeitenDiabetes Case Study - Jason Baoapi-522847737Noch keine Bewertungen
- Cortisol and AsthmaDokument5 SeitenCortisol and AsthmaMin-Joo Esther ParkNoch keine Bewertungen
- Obesity Is A Medical Condition in Which Excess Body FatDokument6 SeitenObesity Is A Medical Condition in Which Excess Body FatgudduzidduNoch keine Bewertungen
- Disorder by PrevalenceDokument13 SeitenDisorder by PrevalenceAbdul Qader BasahamNoch keine Bewertungen
- The Diabetes and The BrainDokument26 SeitenThe Diabetes and The BrainPrecious SantayanaNoch keine Bewertungen
- Benefits of ProbioticsDokument2 SeitenBenefits of ProbioticsGeo IuliaNoch keine Bewertungen
- Probiotics Research PaperDokument12 SeitenProbiotics Research Paperapi-271090706Noch keine Bewertungen
- Oxidative Damage, Aging and Anti-Aging Strategies PDFDokument17 SeitenOxidative Damage, Aging and Anti-Aging Strategies PDFsarahNoch keine Bewertungen
- Effects of Hormones On Metabolic RateDokument6 SeitenEffects of Hormones On Metabolic RatemjfphotoNoch keine Bewertungen
- Hydration FlyerDokument2 SeitenHydration FlyerKatrina Nelson100% (1)
- Mediterranean Diet Cookbook for Beginners: Unlock the Health Benefits of the Mediterranean Diet with Easy and Delicious Recipes for Everyday Eating!Von EverandMediterranean Diet Cookbook for Beginners: Unlock the Health Benefits of the Mediterranean Diet with Easy and Delicious Recipes for Everyday Eating!Noch keine Bewertungen
- Protein Digestion Absorption NASPGHANDokument4 SeitenProtein Digestion Absorption NASPGHANDahiru MuhammadNoch keine Bewertungen
- Probiotics and Prebiotics Health ClaimDokument3 SeitenProbiotics and Prebiotics Health ClaimPhilipNoch keine Bewertungen
- Presentation On Hydration PDFDokument17 SeitenPresentation On Hydration PDFManuel CastroNoch keine Bewertungen
- Gut Microbiome Metabolic Health Microba September 2020Dokument41 SeitenGut Microbiome Metabolic Health Microba September 2020nabilahstsmyhNoch keine Bewertungen
- Digestion Lesson 6Dokument9 SeitenDigestion Lesson 6api-300965210Noch keine Bewertungen
- Key Advances in Medicine 2015Dokument98 SeitenKey Advances in Medicine 2015Alain Riveros100% (2)
- Collagen and GelatinDokument10 SeitenCollagen and GelatinDewi S. GadiNoch keine Bewertungen
- Breathing ExerciseDokument2 SeitenBreathing ExerciseDinesh KumarNoch keine Bewertungen
- Diet and Dermatology: The Role of Dietary Intervention in Skin DiseaseDokument6 SeitenDiet and Dermatology: The Role of Dietary Intervention in Skin Diseaseartventoure projectNoch keine Bewertungen
- Introduction To Corrective Health MedicineDokument107 SeitenIntroduction To Corrective Health MedicineBradford S. Weeks100% (2)
- LDN Information (2!19!17 Update)Dokument18 SeitenLDN Information (2!19!17 Update)bktango100% (1)
- Sarna Thyroid MMC SIBO ConnectionDokument62 SeitenSarna Thyroid MMC SIBO ConnectionGirija VojtkóNoch keine Bewertungen
- Chlorine Dioxide Do You Really Need It This Will Help You Decide!Dokument8 SeitenChlorine Dioxide Do You Really Need It This Will Help You Decide!Pristine WaterNoch keine Bewertungen
- Chinese Medicine For Next Generation HealthcareDokument11 SeitenChinese Medicine For Next Generation Healthcarenina_zheng8244Noch keine Bewertungen
- Drug-Herb InteractionsDokument18 SeitenDrug-Herb InteractionsRoselyn DawongNoch keine Bewertungen
- GelatinDokument3 SeitenGelatinMutiara ParisaNoch keine Bewertungen
- J Food Sci Technol, 2014, 51, 2271-2288Dokument18 SeitenJ Food Sci Technol, 2014, 51, 2271-2288SonaliNoch keine Bewertungen
- Coral CalciumDokument5 SeitenCoral CalciumRama ShaktiNoch keine Bewertungen
- ARW Hexagon 100610Dokument52 SeitenARW Hexagon 100610Violet Lee100% (1)
- Lecture 24 Part 1 - Nutritional Suppliment Part-1-2 PDFDokument25 SeitenLecture 24 Part 1 - Nutritional Suppliment Part-1-2 PDFSYED ALI AHMED KAZMI TMANoch keine Bewertungen
- Immunity and Obesity and Insulin ResistanceDokument9 SeitenImmunity and Obesity and Insulin ResistancePako MorenoNoch keine Bewertungen
- Gelatin Alternatives For The Food IndustryDokument5 SeitenGelatin Alternatives For The Food Industrymdsanchezo8373Noch keine Bewertungen
- DetoxDokument6 SeitenDetoxAline CruzNoch keine Bewertungen
- ResveratrolDokument5 SeitenResveratrolBurlucFlorinNoch keine Bewertungen
- Antibacterial Activity of Propolis Produced by Trigona Spp. Against Campylobacter SPPDokument4 SeitenAntibacterial Activity of Propolis Produced by Trigona Spp. Against Campylobacter SPPAmin FatoniNoch keine Bewertungen
- Allergy Diagnostic TestingDokument149 SeitenAllergy Diagnostic TestingArrow Ou100% (1)
- The Human Microbiome at The Interface of Health and Disease PDFDokument11 SeitenThe Human Microbiome at The Interface of Health and Disease PDFabcder1234Noch keine Bewertungen
- Hyperbaric Oxygen Therapy Indications, Contraindictions and ComplicationsDokument5 SeitenHyperbaric Oxygen Therapy Indications, Contraindictions and ComplicationsMark DingleNoch keine Bewertungen
- Eat Right, Live Well Understanding the Science of NutritionVon EverandEat Right, Live Well Understanding the Science of NutritionNoch keine Bewertungen
- Pharmacology of Pituitary HormonesDokument54 SeitenPharmacology of Pituitary HormonesAmanuel MaruNoch keine Bewertungen
- Secret Detox Drink Recipe (A Natural Detox Drink Recipe) : IngredientsDokument7 SeitenSecret Detox Drink Recipe (A Natural Detox Drink Recipe) : IngredientsBilal Masood100% (1)
- Berberine A Comprehensive Medicinal and Pharmaceutical ReviewDokument4 SeitenBerberine A Comprehensive Medicinal and Pharmaceutical ReviewSaurabh BhattacharyaNoch keine Bewertungen
- HEALTHDokument12 SeitenHEALTHshobha75100% (2)
- Gastritis: Ns. M. Ali Hamid, M. Kes., CWCCADokument86 SeitenGastritis: Ns. M. Ali Hamid, M. Kes., CWCCAindri damayantiNoch keine Bewertungen
- Role of Magnesium and CalciumDokument5 SeitenRole of Magnesium and CalciumsemiNoch keine Bewertungen
- Introduction To NutritionDokument206 SeitenIntroduction To NutritionMompati LetsweletseNoch keine Bewertungen
- Ortoic AcidDokument25 SeitenOrtoic AcidSandy Wasfy100% (1)
- Natural ProductsDokument8 SeitenNatural ProductsPraveen T MNoch keine Bewertungen
- WheatgrassDokument11 SeitenWheatgrassRadhika Shirke100% (1)
- Frenkel Homeopathy and Cancer 2015Dokument7 SeitenFrenkel Homeopathy and Cancer 2015Para EmhNoch keine Bewertungen
- Thorn Eh and OutDokument24 SeitenThorn Eh and OutNick MaxNoch keine Bewertungen
- The Rise and Fall of High Fructose Corn Syrup and Fibromyalgia: Ending Fibromyalgia Without Drugs or ViolenceVon EverandThe Rise and Fall of High Fructose Corn Syrup and Fibromyalgia: Ending Fibromyalgia Without Drugs or ViolenceNoch keine Bewertungen
- Role of Macronutrients, Micronutrients and Animal FoodsDokument7 SeitenRole of Macronutrients, Micronutrients and Animal FoodsPrathiksha BhatNoch keine Bewertungen
- Codex Guidelines For Bottled WaterDokument4 SeitenCodex Guidelines For Bottled WaterPrabath De SilvaNoch keine Bewertungen
- 8.1-8.6 Redox ReactionsDokument65 Seiten8.1-8.6 Redox ReactionsberonelleNoch keine Bewertungen
- X X X X: Element Electron Arrangement of AtomDokument5 SeitenX X X X: Element Electron Arrangement of AtomAlifah SalwaNoch keine Bewertungen
- Electrical Wiring Commercial Canadian 7th Edition Mullin Solutions ManualDokument35 SeitenElectrical Wiring Commercial Canadian 7th Edition Mullin Solutions Manualspinifexcandock8zf100% (24)
- Tiamina, IPDokument4 SeitenTiamina, IPmagicianchemistNoch keine Bewertungen
- ISO/WD xxxxx-1: © ISO 2010 - All Rights ReservedDokument19 SeitenISO/WD xxxxx-1: © ISO 2010 - All Rights ReservedAnanda Ganesh MadheswaranNoch keine Bewertungen
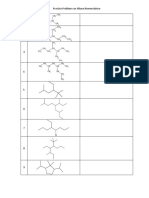
- Practice Problems On Alkane Nomenclature: CH CHDokument2 SeitenPractice Problems On Alkane Nomenclature: CH CHRishav Sasmal100% (2)
- Chapter2 ElectronicEffectsDokument63 SeitenChapter2 ElectronicEffectsMinhh NguyễnNoch keine Bewertungen
- DR - Sajjad Haider: Cambridge International AS & A LevelDokument16 SeitenDR - Sajjad Haider: Cambridge International AS & A Level탁서연Noch keine Bewertungen
- This Study Resource Was: Bgmea University of Fashion and TechnologyDokument9 SeitenThis Study Resource Was: Bgmea University of Fashion and TechnologyRj SajolNoch keine Bewertungen
- Baek 1985Dokument4 SeitenBaek 1985FELIPE DANIEL MONTERO BRUNINoch keine Bewertungen
- Chemistry 2 QuestionsDokument23 SeitenChemistry 2 QuestionsRozilah YunusNoch keine Bewertungen
- 97 03 EDokument28 Seiten97 03 EAgustin CesanNoch keine Bewertungen
- GAS LEL and UELDokument4 SeitenGAS LEL and UELrajesh4dearsNoch keine Bewertungen
- Alkaloids IntroductionDokument44 SeitenAlkaloids IntroductionSyed A RazaNoch keine Bewertungen
- Benzalkonium Chloride PDFDokument1 SeiteBenzalkonium Chloride PDFJay PatelNoch keine Bewertungen
- Biology For The IB Diploma Chapter 2 SummaryDokument8 SeitenBiology For The IB Diploma Chapter 2 SummaryJulian WolfgangNoch keine Bewertungen
- Introduction To AmmoniaDokument15 SeitenIntroduction To AmmoniaHameed Akhtar100% (1)
- Sample: Acidic Exposure - H2SO4 - TM 004Dokument5 SeitenSample: Acidic Exposure - H2SO4 - TM 004G.W.S.S.B SUB DVISION2 MORBINoch keine Bewertungen
- Tixogel Ez 100 TDS (En)Dokument2 SeitenTixogel Ez 100 TDS (En)optimus_1404Noch keine Bewertungen
- IODINE NUMBER (Wijs Method)Dokument4 SeitenIODINE NUMBER (Wijs Method)Visarika Vaidya100% (1)
- Chelate Effect (Recovered)Dokument13 SeitenChelate Effect (Recovered)Ishu AttriNoch keine Bewertungen
- CarbohydratesDokument31 SeitenCarbohydratesMarlene GonsalvezNoch keine Bewertungen
- Soap ReportDokument16 SeitenSoap ReportAddison JuttieNoch keine Bewertungen
- SurfaktanDokument3 SeitenSurfaktanrainbowrhuNoch keine Bewertungen
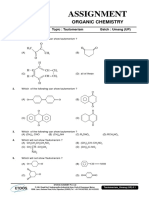
- Assignment: Organic ChemistryDokument6 SeitenAssignment: Organic ChemistryWalid EbaiedNoch keine Bewertungen
- Guide For M SC Inorganic Chemistry Practicals PDFDokument11 SeitenGuide For M SC Inorganic Chemistry Practicals PDFAdil MehboobNoch keine Bewertungen
- Staining - Practical and Theoretical (1962) by GurrDokument654 SeitenStaining - Practical and Theoretical (1962) by GurrDan JohnsonNoch keine Bewertungen
- Sulfur Measurement HandbookDokument21 SeitenSulfur Measurement HandbookcholbertNoch keine Bewertungen
- Catalytic Reaction EngineeringDokument48 SeitenCatalytic Reaction EngineeringM Deepika100% (1)
- Sulfate AttackDokument19 SeitenSulfate AttackIrvebry Ayu WulandaryNoch keine Bewertungen