Beruflich Dokumente
Kultur Dokumente
Reaction List v002
Hochgeladen von
cecil3414Originalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Reaction List v002
Hochgeladen von
cecil3414Copyright:
Verfügbare Formate
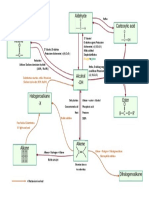
List of organic reactions Number 1 2 Reaction Combustion Free Radical Substitution Reactant Alkane + O2 Alkane + Cl2 or Br2 Product
CO2 and H2O Alkyl Halides + HX Condition / Catalyst In the presence of sunlight or uv. Does not proceed in the dark at room temperature Zeolite, 450 degree C 450 to 700 degree C, 70 atm Ni, 150 degree C Markovnikoffs rule. H3PO4, 60 atm, 300 0C Gaseous X2 room temperature Aqueous X2 room temperature Room temperature, dark Room temperature, dark Room temperature, alkaline, cold Acidic hot concentrated KMnO4.
3 4 5 6 7 8 9 10 11 12
Catlytic Cracking Thermal Cracking Addition of hydrogen, hydrogenation Addition of steam, hydration Addition of hydrogen halides Addition of hydrogen halides Addition of halogen Addition of halogen Mild oxidation Vigorous oxidation
Alkane Alkane Alkene + H2(g) Alkene + H2O(g) Alkene + HX(g) Alkene + HX(aq) Alkene + X2 in CCl4 Alkene + X2(aq) Alkene + MnO4Alkene + MnO4-
Alkane + alkene + H2 Alkane + alkene, H2 Alkane Alcohol Alkyl halides Alkyl halides, alcohol halides. Alkyl halides + HX Alkyl halides + HX, Might contain OH Alkandiol CO2 + H2O, Or ketone, Or carboxylic acid Addition polymer Halogeno benzene + HX (steamy fumes) Nitrobenzene (yellow oil) 1,3,5, trinitrobenzene 2-nitro methyl benzine or 4-nitro methyl benzene Trinitro Toluene (pale yellow solid) Benzoic acid + carboxylic acids or ketone or CO2
13 14
Addition polymerisation Halogenation of benzene Electrophillic substitution Nitration of benzene Dinitration, trinitation of benzene Nitration of methyl benzene Trinitation of methyl benzene Oxidation of side chain
alkene Benzene ring + X2
FeCl3 or FeBr3 or AlCl3 or AlBr3 in the dark Conc H2SO4, Below 50 0C Conc H2SO4, above 60 0C Conc H2SO4, 30 0C
15 16 17
Benzene ring + HNO3 Benzene ring + HNO3 Methyl benzene + HNO3 Methyl benzene + HNO3 benzene ring + KMnO4
18 19
Conc H2SO4, 100 0C Hot acidified KMnO4
www.singaporestudent.com Page | 1
Number 20 21
Reaction Hydrolysis Elimination
22 23 24 25 26 27 28 29 30 31 32 33 34 35
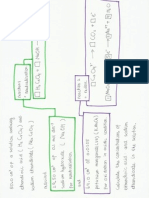
Formation of nitrile Acidic Hydrolysis Alkaline Hydrolysis Reduction of nitrile Reduction of nitrobenzene Formation of 10 amines Formation of 20 amines Formation of 30 amines Formation of 40 amines Combustion Nucleophillic Substitution Nucleophillic Substitution Nucleophillic Substitution Nucleophillic Substitution Test for aliphatic alcohol Nucleophillic Substitution Test for aliphatic alcohol Test for phenol Test for phenol Test for phenol
Reactant Alkyl halides + KOH or NaOH (aq) Alkyl halides + Ethanolic KOH or NaOH Alkyl halides + KCN Nitrile + dilute HCl Nitrile + dilute NaOH Nitrile + LiAlH4 Sn + excess HCl + NaOH Alkyl halides + NH3 Alkyl halides + 10 amines Alkyl halides + 20 amines Alkyl halides + 30 amines Alcohol + O2 Alcohol + HX Alcohol + HX Alcohol + PI3 Alcohol + PCl5
Product Alcohol + HX Alkene + H2O + KX
Condition / Catalyst Heat or reflux Heat or reflux
Alkanitrile + HX Carboxylic acid + NH4Cl Sodium carboxylate + NH3 Amines Benzenamines HX + 10 amines HX + 20 amines HX + 30 amines HX + 40 amines
Ethanolic KCN, heat or reflux Boiling acid Boiling alkali LiAlH4 in ether
Heat in sealed tube, conc NH3 in ethanol. Heat in sealed tube, conc NH3 in ethanol. Heat in sealed tube, conc NH3 in ethanol. Heat in sealed tube, conc NH3 in ethanol.
36
Alcohol + SOCl2
37 38 39
Phenol + PCl5 Phenol + SOCl2 Phenol+ FeCl3
40 41
Test for enol Test for aliphatic alcohol
Phenol+ FeCl3 Aliphatic alcohol+ FeCl3
CO2 + H2O Alkyl halides + H2O Refluxed with NaX and conc H2SO4. X= Cl or Br Alkyl halides + H2O Refluxed with NaX and conc H3PO4. X= I Alkyl halides + Red phosporous and Iodine H3PO3 Alkyl halides + HCl (steamy fumes) + POCl3 Alkyl halides + SO2+ HCl(steamy fumes) NO REACTION NO REACTION C6H5O-FeCl2 = Neutral solution of FeCl3 purple solution + HCl RO-FeCl2 = purple Neutral solution of FeCl3 solution + HCl NO REACTION
www.singaporestudent.com Page | 2
Number 42 43 44 45 46 50 51 52 53 54 55
Reaction Acid metal Oxidation of 10 alcohol Oxidation of 10 alcohol Oxidation of 20 alcohol Oxidation of 30 alcohol Dehydration Esterification Esterification Acylation Acylation Tri Iodo methane
Reactant Alcohol + Na(s) 10 Alcohol + KMnO4 or K2Cr2O7 10 Alcohol + KMnO4 or K2Cr2O7 20 Alcohol + KMnO4 or K2Cr2O7 30 Alcohol + KMnO4 or K2Cr2O7 Alcohol Alcohol + Carboxylic Acid Phenol + Carboxylic Acid Alcohol + Acyl chloride Phenol + Acyl chloride Methyl alcohol + NaOH(aq) + I2(aq) Aliphatic alcohol, NaOH Phenol + NaOH Aliphatic alcohol + Na Phenol + Na Aliphatic alcohol + Na2CO3 Phenol + Na2CO3 Phenol + HNO3 Phenol + HNO3
Product Sodium alkoxide + H2(g) Aldehyde+ H2O Carboxylic acid+ H2O Ketone + H2O NO REACTION Alkene + H2O Ester + H2O NO REACTION Ester + HCl Phenate Ester + HCl Sodium carboxylate + CHI3 (yellow crystal) NO REACTION Sodium phenoxide + H2O Sodium alkoxide + H2(g) Sodium phenoxide + H2(g) NO REACTION NO REACTION 2-nitro phenol Or 4-nitrophenol 2,4,6 trinitro phenol = picric acid 2,4,6 tribromo phenol = white ppt 2-bromo phenol or 4-bromo phenol + HBr (steamy fumes)
Condition / Catalyst
distill Reflux Distill or reflux
Saytzevs rule, 170 0C,conc H2SO4 Reflux ,conc H2SO4
Room temperature Room temperature warm
56 58 59 60 61 62 63 64
Acid base reaction Acid base reaction Acid metal reaction Acid metal reaction Acid carbonate reaction Acid carbonate reaction Mononitration of phenol Trinitration of phenol
Room temperature, dilute HNO3 Room temperature, conc HNO3
65
Tri-bromination of phenol
Phenol+ Br2(aq)
Room temperature
66
Mono-bromination of phenol
Phenol + Br2 in CCl4
Room temperature
www.singaporestudent.com Page | 3
Number 67 68 69 70 71 72 73 74
Reaction Reduction of aldehyde Reduction of aldehyde Reduction of ketone Reduction of ketone Oxidation of aldehyde Oxidation of ketone Reaction with HCN Test of carbonyl
Reactant Aldehyde Aldehyde Ketone Ketone Aldehyde Ketone + K2Cr2O7 Carbonyl + HCN Carbonyl + 24DNPH
Product 10 Alcohol + H2O 10 Alcohol + H2O 20 Alcohol + H2O 20 Alcohol + H2O Carboxylic acid + H2O NO REACTION Cyanohydrine = hydroxinitrile 24 dinitrophenyl hydrazone = orange crystal Cu2O = red ppt + Carboxylic acid. NO REACTION
Condition / Catalyst LiAlH4 H2 and Ni catalyst LiAlH4 H2 and Ni catalyst KMnO4 or K2Cr2O7, Heat or reflux
In the cold, 10 to 20 0C, trace amount of NaOH or NaCN. Warm
75
Fehling test
76
Fehling test
77
Tollen test
Aliphatic aldehyde + Fehlings solution= Cu2+ Benzaldehyde + Fehlings solution= Cu2+ Aliphatic aldehyde, Tollens reagent = Ag(NH3)2 Benzaldehyde+ Tollens reagent Methyl ketone + NaOH+ I2 Carboxylic acid +NaOH or KOH(aq) Carboxylic acid +NH3 Carboxylic acid + amines Carboxylic acid +Na2CO3 or K2CO3(aq) Carboxylic acid + Na(s) Carboxylic acid + alcohol Carboxylic acid + PCl5
Ag = silver mirror + carboxylic acid
78 79
Tollen test Iodoform test
80 81 82 83 84 85 86
Neutralisation Neutralisation Neutralisation Acid carbonate Acid metal Esterification Formation of acyl chloride
Ag = silver mirror + benzoic acid CHI3 = yellow crystal + carboxylic acid Carboxylate + H2O 10 amide + H2O 20 amide + H2O Carboxylate + CO2 + H2O Carboxylate + H2 Ester + H2O Acyl chloride + POCl3 + HCl = steamy fumes
Warm
Reflux in conc H2SO4 Room temperature
www.singaporestudent.com Page | 4
Number 87
Reaction Formation of acyl chloride
Reactant Carboxylic acid + SOCl2 Acyl cholride + H2O(l) Acyl cholride + aliphatic alcohol Acyl cholride + phenol Acyl cholride + NaOH or KOH Acyl cholride + NH3 Acyl cholride + amines Ester + dilute HCl Ester + dilute NaOH Aliphatic amines + Br2(aq) Benzenamines + Br2(aq) Amides + dilute HCl Amide + dilute NaOH Amino acid Proteins Proteins
88 89 91 92 93 94 95 96 97 98 99 100 101 102 103
Hydrolysis Esterification Esterification Neutralisation Neutralisation Neutralisation Acidic Hydrolyisis Alkaline Hydrolyisis Bromination of amines Bromination of benzenamines Acidic Hydrolyisis Alkaline Hydrolyisis Condensation polymerisation Acidic Hydrolyisis Alkaline Hydrolyisis
Product Acyl chloride + SO2 + HCl = steamy fumes Carboxylic acid + H2O Ester + HCl Ester + HCl Carboxylate + H2O 10 amide + H2O 20 amide + H2O Carboxylic acid + alcohol Carboxylate + alcohol NO REACTION 2,4,6, tribromo benzenamine Carboxylic acid + amines Carboxylate + amines Protein Amino acids Amino acids
Condition / Catalyst Room temperature
Room temperature Room temperature Room temperature Room temperature Room temperature Reflux with dilute HCl Reflux with dilute NaOH
Room temperature Reflux with dilute HCl Reflux with dilute NaOH
Reflux with dilute HCl Reflux with dilute NaOH
www.singaporestudent.com Page | 5
Das könnte Ihnen auch gefallen
- Mod 4 Revision Guide 10 Synthetic RoutesDokument2 SeitenMod 4 Revision Guide 10 Synthetic RoutesdufraiscNoch keine Bewertungen
- Organic-Chemistry (As Level)Dokument8 SeitenOrganic-Chemistry (As Level)Pirate HunterNoch keine Bewertungen
- Organic Chem ReactionsDokument7 SeitenOrganic Chem ReactionsTeo Jia Ming NickolasNoch keine Bewertungen
- Catholic Junior College H2 Chemistry 9729 2019 Practical Handbook - Part 6Dokument13 SeitenCatholic Junior College H2 Chemistry 9729 2019 Practical Handbook - Part 6Timothy HandokoNoch keine Bewertungen
- 2014 YJC Prelim H2 Chem P1 W AnsDokument18 Seiten2014 YJC Prelim H2 Chem P1 W AnswaimoeNoch keine Bewertungen
- Balancing EquationsDokument1 SeiteBalancing Equationschong56Noch keine Bewertungen
- Relationship between metal reactivity and carbonate decompositionDokument3 SeitenRelationship between metal reactivity and carbonate decompositionZou JunyiNoch keine Bewertungen
- A2 Test 11 Notes - Transition ElementsDokument11 SeitenA2 Test 11 Notes - Transition Elementswill bellNoch keine Bewertungen
- 6carboxylic AcidsDokument1 Seite6carboxylic AcidssharmimiameerasanadyNoch keine Bewertungen
- JPJC 2020 JC1 H2 Chemistry Tutorial on Chemical EnergeticsDokument13 SeitenJPJC 2020 JC1 H2 Chemistry Tutorial on Chemical EnergeticsSalman ShethNoch keine Bewertungen
- 3 Experiment ChemistryDokument30 Seiten3 Experiment ChemistryThangavel SarujanNoch keine Bewertungen
- Chemistry PracticalDokument16 SeitenChemistry PracticalmayashankarjhaNoch keine Bewertungen
- Unit 5 Organic Chemistry ReactionsDokument9 SeitenUnit 5 Organic Chemistry ReactionsRobbing_Hood100% (1)
- 2015 H2 Carbonyl Cpds Tutorial Answer Updated PDFDokument25 Seiten2015 H2 Carbonyl Cpds Tutorial Answer Updated PDFJohnNoch keine Bewertungen
- 11 Test Cations AnionsDokument3 Seiten11 Test Cations Anionsapi-27085921100% (1)
- Edexcel IAS Bonding 1Dokument14 SeitenEdexcel IAS Bonding 1mostafa barakatNoch keine Bewertungen
- Reactions of Aldehydes and Ketones: Oxidation Reduction Nucleophilic AdditionDokument51 SeitenReactions of Aldehydes and Ketones: Oxidation Reduction Nucleophilic AdditionmacybnzNoch keine Bewertungen
- Chemistry Practical Test Guide For Cations and AnionsDokument2 SeitenChemistry Practical Test Guide For Cations and Anionsansherina2100% (1)
- 2011 H2 Chem SRJC Prelim Paper 2 Suggested AnswersDokument15 Seiten2011 H2 Chem SRJC Prelim Paper 2 Suggested AnswersonnoezNoch keine Bewertungen
- As Chemistry Organic MindmapDokument1 SeiteAs Chemistry Organic MindmapDương Thị Ngọc HiềnNoch keine Bewertungen
- Hydrocarbons NotesDokument13 SeitenHydrocarbons NotesShivansh Pundir100% (1)
- 14.hydroxyl Compounds Lecture NotesDokument22 Seiten14.hydroxyl Compounds Lecture Notesgeoboom12100% (4)
- Aldehydes and Ketones ReactionsDokument9 SeitenAldehydes and Ketones ReactionsKudzayi Tusaumwe100% (1)
- KSP Calcium Hydroxide C12!4!13Dokument7 SeitenKSP Calcium Hydroxide C12!4!13Nazihah Moomoo50% (2)
- WWW - Crackjee.xyz: Organic ChemistryDokument9 SeitenWWW - Crackjee.xyz: Organic ChemistryRau100% (1)
- Section A: Mcqs Halogen DerivativesDokument11 SeitenSection A: Mcqs Halogen DerivativesBint A. Qadir100% (1)
- Redox RxnsDokument30 SeitenRedox RxnsJolaine ValloNoch keine Bewertungen
- Aqueous Ion Colors: AP Chemistry: Colors Flame Test ColorsDokument1 SeiteAqueous Ion Colors: AP Chemistry: Colors Flame Test ColorsZhi ZhingNoch keine Bewertungen
- Science (Chemistry) SA PracticeDokument4 SeitenScience (Chemistry) SA Practicechong56Noch keine Bewertungen
- Carbonyl Compounds: Aldehydes, Ketones and Carboxylic AcidsDokument16 SeitenCarbonyl Compounds: Aldehydes, Ketones and Carboxylic AcidsRanit Mukherjee100% (1)
- 100 Organic Reagentspptx - 230327 - 085539 PDFDokument15 Seiten100 Organic Reagentspptx - 230327 - 085539 PDFHeera MeenaNoch keine Bewertungen
- H2 Chem Summary of Chemical PeriodicityDokument7 SeitenH2 Chem Summary of Chemical Periodicityonnoez100% (2)
- 9791 Chemistry Example Candidate Responses Booklet WEBDokument129 Seiten9791 Chemistry Example Candidate Responses Booklet WEByvcgNoch keine Bewertungen
- IB Chem 1 Assess Cws1aDokument3 SeitenIB Chem 1 Assess Cws1aEmi JiHyeon KimNoch keine Bewertungen
- Everything You Need to Know About SaltsDokument32 SeitenEverything You Need to Know About SaltsSabriza Hassan Assa'ariNoch keine Bewertungen
- Detailed Notes For ch4Dokument24 SeitenDetailed Notes For ch4Jemima KaishaNoch keine Bewertungen
- Halogens Information SheetDokument4 SeitenHalogens Information Sheetmallika29Noch keine Bewertungen
- Name ReactionsDokument10 SeitenName ReactionsParam SoniNoch keine Bewertungen
- Organic reactions of carbonyl compoundsDokument3 SeitenOrganic reactions of carbonyl compoundsmohdburhantalatNoch keine Bewertungen
- CIE Chemistry Revision Guide For A2 LevelDokument15 SeitenCIE Chemistry Revision Guide For A2 LevelBakhita MaryamNoch keine Bewertungen
- OCR Chemistry NotesDokument10 SeitenOCR Chemistry NotesJack WoodNoch keine Bewertungen
- Sec 4 Chemistry PracticeDokument4 SeitenSec 4 Chemistry Practicechong56Noch keine Bewertungen
- t2 Chem Revision Ex 22 - Answer SchemeDokument20 Seitent2 Chem Revision Ex 22 - Answer SchemeNicholas Ow50% (2)
- Equilibria Questions and Answers For A2 ChemistryDokument303 SeitenEquilibria Questions and Answers For A2 ChemistrybloodymerlinNoch keine Bewertungen
- Redox ReactionsDokument29 SeitenRedox ReactionsSoniaAlexNoch keine Bewertungen
- AS Level Qualitative AnalysisDokument8 SeitenAS Level Qualitative AnalysismahahajNoch keine Bewertungen
- Chemistry Required Practical 3Dokument4 SeitenChemistry Required Practical 3tiaNoch keine Bewertungen
- Chap5 IGCSE Chemistry NotesDokument13 SeitenChap5 IGCSE Chemistry NotesMisbah Kamran0% (1)
- JC 2 PRELIM EXAM CHEMISTRY ANSWERSDokument25 SeitenJC 2 PRELIM EXAM CHEMISTRY ANSWERSYeeloong YlNoch keine Bewertungen
- Organic ReagentsDokument3 SeitenOrganic ReagentsKushagra Rai100% (1)
- 2012 OLevel Science Chemistry Paper 3 Questions and AnswersDokument10 Seiten2012 OLevel Science Chemistry Paper 3 Questions and AnswersMethodology OfStudies100% (1)
- JEE - Haloalkanes & Haloarenes - (Q+S)Dokument13 SeitenJEE - Haloalkanes & Haloarenes - (Q+S)Sachin DedhiaNoch keine Bewertungen
- 7 Coordination CompoundsDokument329 Seiten7 Coordination CompoundsArka100% (1)
- Electrolysis QuestionsDokument53 SeitenElectrolysis QuestionsAahaan ShethNoch keine Bewertungen
- Electroysis WorksheetDokument2 SeitenElectroysis WorksheetericaNoch keine Bewertungen
- Viva Questions Salt Anlysis and Functional GroupDokument4 SeitenViva Questions Salt Anlysis and Functional GroupWill The WiseNoch keine Bewertungen
- Identification of Cations, Anions and GasesDokument2 SeitenIdentification of Cations, Anions and GasesMustufa FerozNoch keine Bewertungen
- 1.aldehydes, Ketones and Carboxylic AcidsDokument117 Seiten1.aldehydes, Ketones and Carboxylic AcidsKRISHNARJUNA NNoch keine Bewertungen
- O Level Biology Practice Questions And Answers: Coordination And ResponseVon EverandO Level Biology Practice Questions And Answers: Coordination And ResponseNoch keine Bewertungen
- Mol Calculation Notes For O Level StudentDokument93 SeitenMol Calculation Notes For O Level Studentcecil3414Noch keine Bewertungen
- Completing The Square v001Dokument5 SeitenCompleting The Square v001cecil3414Noch keine Bewertungen
- Completing The Square v001Dokument5 SeitenCompleting The Square v001cecil3414Noch keine Bewertungen
- Trend GraphDokument1 SeiteTrend Graphcecil3414Noch keine Bewertungen
- c07 U09 PH of All SolutionDokument4 Seitenc07 U09 PH of All Solutioncecil3414Noch keine Bewertungen
- Volumetric Analysis Exercise v002Dokument3 SeitenVolumetric Analysis Exercise v002cecil3414Noch keine Bewertungen
- Volumetric Analysis ExerciseDokument3 SeitenVolumetric Analysis Exercisececil3414Noch keine Bewertungen
- Type of SubstancesDokument3 SeitenType of Substancescecil3414100% (1)
- Trend GraphDokument7 SeitenTrend Graphcecil3414Noch keine Bewertungen
- Answer KeyDokument2 SeitenAnswer Keycecil3414Noch keine Bewertungen
- c01 StoichiometryDokument14 Seitenc01 Stoichiometrycecil3414Noch keine Bewertungen
- List of Irreversible ReactionsDokument12 SeitenList of Irreversible Reactionscecil3414Noch keine Bewertungen
- MCQDokument6 SeitenMCQcecil3414Noch keine Bewertungen
- c02 Atomic StructureDokument17 Seitenc02 Atomic Structurececil3414Noch keine Bewertungen
- c04 States of MatterDokument19 Seitenc04 States of Mattercecil3414Noch keine Bewertungen
- MCQDokument2 SeitenMCQcecil3414Noch keine Bewertungen
- MCQDokument4 SeitenMCQcecil3414Noch keine Bewertungen
- MCQDokument4 SeitenMCQcecil3414Noch keine Bewertungen
- MCQDokument2 SeitenMCQcecil3414Noch keine Bewertungen
- MCQDokument6 SeitenMCQcecil3414Noch keine Bewertungen
- Self-Balancing Two-Wheeler Using GyroscopeDokument33 SeitenSelf-Balancing Two-Wheeler Using Gyroscopemilan mottaNoch keine Bewertungen
- List of Computer Networking DevicesDokument1 SeiteList of Computer Networking Deviceskamit17102900100% (1)
- Datasheet De14h (II) HC 1500v May2019 NTDokument2 SeitenDatasheet De14h (II) HC 1500v May2019 NTkrishnakumar paamireddyNoch keine Bewertungen
- Metric Heavy Hex Nuts: ASME B18.2.4.6M-2010Dokument16 SeitenMetric Heavy Hex Nuts: ASME B18.2.4.6M-2010CarlitosNoch keine Bewertungen
- Borneo SporenburgDokument2 SeitenBorneo SporenburgDorin TecuceanuNoch keine Bewertungen
- Purification of Morphologically and Functionally Intact Human Basophils To Near HomogeneityDokument9 SeitenPurification of Morphologically and Functionally Intact Human Basophils To Near HomogeneitySinaí GutierrezNoch keine Bewertungen
- 2021 ESC Guidelines For The Diagnosis and Treatment of Acute and Chronic Heart FailureDokument137 Seiten2021 ESC Guidelines For The Diagnosis and Treatment of Acute and Chronic Heart FailuredianNoch keine Bewertungen
- Tramadol Drug StudyDokument1 SeiteTramadol Drug Studymilkv82% (11)
- Chemistry How To Make Stuff PDFDokument184 SeitenChemistry How To Make Stuff PDF2967449CEENoch keine Bewertungen
- ASME B31.4-2016 Pipeline Transportation Systems For Liquids and SlurriesDokument1 SeiteASME B31.4-2016 Pipeline Transportation Systems For Liquids and SlurriesJose Rodrigo Salguero DuranNoch keine Bewertungen
- CIVL-365 Tutorial 8 SolutionDokument3 SeitenCIVL-365 Tutorial 8 SolutionIvsNoch keine Bewertungen
- Eplob Eplob/A Epmob Epmob/A: PhotocellsDokument2 SeitenEplob Eplob/A Epmob Epmob/A: PhotocellsSupuran RichardoNoch keine Bewertungen
- CORRELATION AND LINEAR REGRESSIONDokument9 SeitenCORRELATION AND LINEAR REGRESSIONSANKET GANDHINoch keine Bewertungen
- 10 01 Breather Filters GBDokument8 Seiten10 01 Breather Filters GBosuengNoch keine Bewertungen
- CERT Basic Training Participant Manual - 2011Dokument332 SeitenCERT Basic Training Participant Manual - 2011jegodfreyNoch keine Bewertungen
- Inakyd 3623-X-70Dokument2 SeitenInakyd 3623-X-70roybombomNoch keine Bewertungen
- Chemical reactions and structuresDokument22 SeitenChemical reactions and structuresStormy StudiosNoch keine Bewertungen
- Data Biostataplus Sbi2014-EDokument4 SeitenData Biostataplus Sbi2014-ELucila Milagros PinillosNoch keine Bewertungen
- Inkontinensia Urin: Dr. Adhi Permana, SPPDDokument35 SeitenInkontinensia Urin: Dr. Adhi Permana, SPPDTiara KhairinaNoch keine Bewertungen
- TICSA - Diesel Uno Petroleos Guatemala (13.01.23)Dokument1 SeiteTICSA - Diesel Uno Petroleos Guatemala (13.01.23)Luis M LópezNoch keine Bewertungen
- Difference Between AerospaceDokument2 SeitenDifference Between AerospaceSyawalMaulanaNoch keine Bewertungen
- Baby NamesDokument9 SeitenBaby Namesppremamca_617705407Noch keine Bewertungen
- Manual of Curatorship: A Guide To Museum PracticeDokument7 SeitenManual of Curatorship: A Guide To Museum PracticeLuísa MenezesNoch keine Bewertungen
- (Computing 14) A. Aguilera, D. Ayala (Auth.), Professor Dr. Guido Brunnett, Dr. Hanspeter Bieri, Professor Dr. Gerald Farin (Eds.) - Geometric Modelling-Springer-Verlag Wien (2001)Dokument356 Seiten(Computing 14) A. Aguilera, D. Ayala (Auth.), Professor Dr. Guido Brunnett, Dr. Hanspeter Bieri, Professor Dr. Gerald Farin (Eds.) - Geometric Modelling-Springer-Verlag Wien (2001)ANDRES Fernando Mosquera DIAZNoch keine Bewertungen
- NNDC Planning Applications 4oct - 11 OctDokument4 SeitenNNDC Planning Applications 4oct - 11 OctRichard SmithNoch keine Bewertungen
- Natural Law Theory ApproachDokument35 SeitenNatural Law Theory ApproachseventhwitchNoch keine Bewertungen
- Classified Advertisements from Gulf Times NewspaperDokument6 SeitenClassified Advertisements from Gulf Times NewspaperAli Naveed FarookiNoch keine Bewertungen
- 20 N 60 C 3Dokument13 Seiten20 N 60 C 3rashidmirzaNoch keine Bewertungen
- Synchronized Natural Incubation by Free-Range Native ChickensDokument2 SeitenSynchronized Natural Incubation by Free-Range Native ChickensFilbert John MillanNoch keine Bewertungen
- Fault Code 155: Intake Manifold Air Temperature High - CriticalDokument3 SeitenFault Code 155: Intake Manifold Air Temperature High - Criticalhamilton miranda100% (1)