Beruflich Dokumente
Kultur Dokumente
Files 2-Experiments Homogenuous Batch Reactor
Hochgeladen von
S M AseemOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Files 2-Experiments Homogenuous Batch Reactor
Hochgeladen von
S M AseemCopyright:
Verfügbare Formate
EXPERIMENT No: RK-1 Homogeneous Batch Reactor-1
Objective:
To determine the order and value of the rate constant for the homogeneous liquid phase reaction of caustic soda with ethyl acetate in a batch reactor:
NaOH + CH 3 COOC2 H 5
C2 H 5OH + CH 3COONa
Introduction
A batch reactor may be described as a vessel in which chemicals are placed to react. Batch reactors are normally used in small-scale laboratory set-ups to study the kinetics of chemical reactions. To determine the order and rate constant of a chemical reaction, the variation of a property of the reaction mixture is observed as the reaction progresses. Data collected usually consist of changes in variables such as concentration of a component, total volume of the system or a physical property like electrical conductivity or refractive index. The data are then analyzed using pertinent equations to find desired kinetic parameters.
Theory For any given reaction in a constant volume system, the rate of the reaction can be represented by:
rA = kf ( C A ) =
dC A dt
(1)
Where rA is the rate of disappearance of reactant A among the reacting species. Equation (1) can be rearranged to give:
dC
A A
f (C )
= kdt
(2)
Integrating equation (2) analytically yields
RK1-1
Af
CA
t dC A = k dt = kt f (C A ) o
(3)
By postulating various forms for f(CA) in equation (3) and correlating the resulting equation with the experimental data, the rate constant k, and order of the reaction can be determined assuming that the rate of reaction can be expressed by an equation of the form:
n ( rA ) = kC A
(4)
The values of n and k can be determined experimentally, and has also been reported in literature.
Procedure
1)
In the reactor (see Figure 1), mix 1.0 liter of the 0.1M caustic soda solution with 1.0 liter of the 0.1M ethyl acetate solution at an arbitrary time (t = 0) at room temperature. Switch on the stirrer immediately and set it to an intermediate speed to avoid splashing. Start the timer as soon as you start mixing the reactants. After a certain time interval, use a pipette to withdraw 25ml sample from the reactor, and immediately quench it with 25ml of excess 0.05M hydrochloric acid.[You should have the quenching acid sample ready before taking the sample from the reactor.] Add 2 - 3 drops of phenolphethalene to the quenched sample and back titrate with 0.05M NaOH solution until the end point is detected (in this case a stable pink color) . Record the amount of NaOH used in the titration (Vtit.). Repeat steps (3) - (5) every 3 minutes for the first five samples and thereafter every 5 minutes. Take a total of 14 samples making sure that you record the time for each new sample.
2) 3)
4)
5) 6)
Note : It is recommended that you prepare the 25ml quenching acid samples in different flasks before starting the reaction.
RK1-2
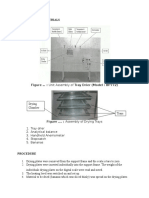
Stirrer
Inlet orifice
Thermometer
Reactor wall
Reaction mixture
Drain valve
Figure 1: Batch Reactor Set-up
RK1-3
Report Requirement
1.
Using the volume of 0.05M NaOH used in the titration, calculate the concentration (in mol/lit) of unreacted NaOH in each sample withdrawn from the reactor. The following equation may be used:
CA =
Note
[ 25.0 - Vtit. ] xCA 0
25.0
(mol/lit)
(5)
You should know the basis of this equation, and that this is not a general formula for all cases. Here CA0 is the concentration of the standard NaOH solution.
2.
Apply the integral method of analysis to determine the reaction order and rate constant of the reaction. This could be done as follows : a. Assume an expression (f(CA)) for the reaction rate (zero-order, 1st order, 2nd order etc.) b. Substitute the expression into equation (3) and integrate. c. Rearrange the resulting expression and plot. Note : all the above steps should be shown in the report
3.
Note
From the literature, find both the order and rate constant of the reaction.
You must mention in your report the exact reference i.e. title, author and page number of the source from which you obtained the values. Also attach a copy of the page containing the information.
4.
Compare between the theoretical and experimental values and give reasons for the deviations.
Nomenclature
CA CAf
= Concentration of A, mol/lit. = Final concentration of reactant A (mol/lit) = Inlet concentration of A, mol/lit = Rate constant for the reaction. = Order of reaction = rate of reaction moles A/lit. sec.
C A0
k n
rA
RK1-4
t V Vtit.
= time, min. = Volume of mixture, lit = Volume of NaOH used in titration (ml)
References
1.
Levenspiel, O., "Chemical Reaction Engineering", 2nd ed., Wiley and Sons, N.Y., p. 41 (1977). Smith, J.M., "Chemical Engineering Kinetics", 3rd ed., McGraw-Hill Book Comp., N.Y., p. 37 (1981). Holland, C. D "An Introduction to Chemical Engineering Kinetics & Reactor Design. " Chp. 8, John Wiley Inc., N.Y., (1977).
2.
3.
RK1-5
LOG SHEET FOR EXPERIMENT RK-1
Names and ID # students:
1: ________________________ ID# __________ 2: ________________________ ID# __________ 3: ________________________ ID# __________
Date: Table 1: Experimental Data Sample # Time (min) 1 2 3 4 5 6 7 8 9 10 11 12 13 14 3 6 9 12 15 20 25 30 35 40 45 50 55 60 Initial Burette reading [R1] (ml) Final Burette reading [R2] (ml) Volume NaOH used in Titration [R2-R1](ml)
Sample Volume = 25ml Volume of 0.05M HCl used in quenching = 25 ml
RK1-6
Das könnte Ihnen auch gefallen
- Practical Chemical Thermodynamics for GeoscientistsVon EverandPractical Chemical Thermodynamics for GeoscientistsNoch keine Bewertungen
- P4E2: Kinetics of Homogeneous Reaction in Batch and Continuous Stirred-Tank Reactor at Two Different TemperatureDokument7 SeitenP4E2: Kinetics of Homogeneous Reaction in Batch and Continuous Stirred-Tank Reactor at Two Different TemperaturejayaprinaNoch keine Bewertungen
- Experiment: Batch Reactor Unit Operations Lab I (CHEGR3787L) Fall 2004Dokument5 SeitenExperiment: Batch Reactor Unit Operations Lab I (CHEGR3787L) Fall 2004Janice YanNoch keine Bewertungen
- Cre 1 IntroductionDokument4 SeitenCre 1 IntroductionEvangeline LauNoch keine Bewertungen
- CSTR 40LDokument16 SeitenCSTR 40LhishamNoch keine Bewertungen
- Vapor Liquid EquilibriumDokument7 SeitenVapor Liquid Equilibriummahbub1332100% (1)
- CHE 435 Liquid-Liquid Extraction Lab ManualDokument24 SeitenCHE 435 Liquid-Liquid Extraction Lab ManualStolen RememberNoch keine Bewertungen
- CH 7 AssignmentDokument3 SeitenCH 7 AssignmentUday Prakash SahuNoch keine Bewertungen
- Exp 4 Batch Evaporative Crystallization PDFDokument9 SeitenExp 4 Batch Evaporative Crystallization PDFmirza farhanNoch keine Bewertungen
- Packed Distillation Column ExperimentDokument20 SeitenPacked Distillation Column ExperimentChan Chun ChenNoch keine Bewertungen
- Lab Report TPP Experiment 3Dokument10 SeitenLab Report TPP Experiment 3Nurul Najwa100% (1)
- Chapter 4Dokument43 SeitenChapter 4aliNoch keine Bewertungen
- CPB30004 Process Dynamics and Control Experiment 5: Heat Exchanger Process Control Lab ReportDokument24 SeitenCPB30004 Process Dynamics and Control Experiment 5: Heat Exchanger Process Control Lab ReportSiti Hajar Mohamed100% (1)
- IYOHA COLLINS 16CF020531 Batch Reactor ReportDokument19 SeitenIYOHA COLLINS 16CF020531 Batch Reactor ReportDavid OvieNoch keine Bewertungen
- Plug Flow Reactor ExperimentDokument16 SeitenPlug Flow Reactor ExperimentN Afiqah RazakNoch keine Bewertungen
- Final Poster PresentationDokument1 SeiteFinal Poster PresentationPinjala AnoopNoch keine Bewertungen
- Duhem's theorem determines equilibrium stateDokument1 SeiteDuhem's theorem determines equilibrium stateZafirahAhmadFauzi100% (1)
- Bernoulli EquationDokument6 SeitenBernoulli EquationMohanadAlrofuNoch keine Bewertungen
- Vle UnitDokument26 SeitenVle UnitAhmad Ifwat50% (2)
- Isothermal CSTR PDFDokument9 SeitenIsothermal CSTR PDFprashant_cool_4_uNoch keine Bewertungen
- Oil Distillation ReportDokument10 SeitenOil Distillation ReportnisasoberiNoch keine Bewertungen
- Batch Distillation ExperimentDokument8 SeitenBatch Distillation ExperimentJonelou CusipagNoch keine Bewertungen
- Thermal Laboratory - Lab Experiment-1Dokument7 SeitenThermal Laboratory - Lab Experiment-1Raj PratyushNoch keine Bewertungen
- CELCHA2 Study GuidesDokument7 SeitenCELCHA2 Study GuidesEsther100% (1)
- LleDokument30 SeitenLlefirstlove_492_736373Noch keine Bewertungen
- Adsorption IsothermDokument4 SeitenAdsorption Isothermahkiujtsw0% (1)
- Intro CSTRDokument6 SeitenIntro CSTREmmanuel PlazaNoch keine Bewertungen
- CRE Lab ManualDokument19 SeitenCRE Lab ManualMayursinh Solanki100% (1)
- Gas UnitDokument4 SeitenGas UnitZalina SamsuddinNoch keine Bewertungen
- Optimized Solids Suspension: Achieving Uniform Dispersion is Critical to Product QualityDokument7 SeitenOptimized Solids Suspension: Achieving Uniform Dispersion is Critical to Product QualitymichsantosNoch keine Bewertungen
- Solved Problem Question (Gas Ab)Dokument2 SeitenSolved Problem Question (Gas Ab)Seruzna IshxNoch keine Bewertungen
- Batch ReactorDokument4 SeitenBatch ReactorFoo Xiao BingNoch keine Bewertungen
- Exp - 2 Bubble Cap Distillation ColumnDokument13 SeitenExp - 2 Bubble Cap Distillation ColumnAdawiyah Al-jufri100% (1)
- Process Control & Instrumentations CEV 544 PrelabDokument9 SeitenProcess Control & Instrumentations CEV 544 PrelabFaradilah Binti Ajma'inNoch keine Bewertungen
- Assignment 1 PDFDokument1 SeiteAssignment 1 PDFRoydia SimanNoch keine Bewertungen
- 7405Dokument8 Seiten7405Ebby OnyekweNoch keine Bewertungen
- Conclusion, Recoomendation, Reffenrence, Lab 2, CHE 485Dokument2 SeitenConclusion, Recoomendation, Reffenrence, Lab 2, CHE 485MOHD MU'IZZ BIN MOHD SHUKRINoch keine Bewertungen
- Experiment: Packed Distillation ColumnDokument4 SeitenExperiment: Packed Distillation Columnnhalieza1067Noch keine Bewertungen
- Lab 1Dokument12 SeitenLab 1JoeJeanNoch keine Bewertungen
- Osbourne ReynoldDokument13 SeitenOsbourne ReynoldN Afiqah Razak0% (1)
- Identifying Flow Type Using Reynolds ApparatusDokument5 SeitenIdentifying Flow Type Using Reynolds ApparatusKonem SolutionsNoch keine Bewertungen
- Isothermal Batch ReactorDokument5 SeitenIsothermal Batch ReactorSrikanthNoch keine Bewertungen
- RI Vs Composition Methanol-Water MixtureDokument12 SeitenRI Vs Composition Methanol-Water MixtureAnonymous VeJYFSMWLINoch keine Bewertungen
- Saperation 1: Ass. Prof. Adnan Ripin Faculty of Chemical and Energy Engineering Universiti Teknologi MalaysiaDokument79 SeitenSaperation 1: Ass. Prof. Adnan Ripin Faculty of Chemical and Energy Engineering Universiti Teknologi MalaysiaNurul AinNoch keine Bewertungen
- Apparatus, Procedure, Recommendation Tray DryerDokument4 SeitenApparatus, Procedure, Recommendation Tray DryerillyzlNoch keine Bewertungen
- 4.3. Consider The Flowsheet For The Manufacture of Vinyl Chloride in Figure 4.8Dokument2 Seiten4.3. Consider The Flowsheet For The Manufacture of Vinyl Chloride in Figure 4.8Anonymous QwUTQlAO100% (1)
- Lab10 CompleteDokument22 SeitenLab10 CompleteMastura Ahmad Termizi100% (1)
- CPI LAB ManualDokument76 SeitenCPI LAB ManualFarhad Iqbal100% (2)
- Experiment 1B - Tubular ReactorDokument14 SeitenExperiment 1B - Tubular ReactorNajmul Puda PappadamNoch keine Bewertungen
- Cooling Tower ReportDokument11 SeitenCooling Tower Reportbae zazNoch keine Bewertungen
- Integrated Cheese PlantDokument5 SeitenIntegrated Cheese PlantMohamedNoch keine Bewertungen
- Ex 1Dokument7 SeitenEx 1yad AGNoch keine Bewertungen
- Exp-40 Part2Dokument22 SeitenExp-40 Part2Ahmet Samet ÖzdilekNoch keine Bewertungen
- Nitric Acid: Created By:-Aman Arya 10 A Class Roll No:-3Dokument8 SeitenNitric Acid: Created By:-Aman Arya 10 A Class Roll No:-3Aman AryaNoch keine Bewertungen
- Lab ReportDokument10 SeitenLab ReportKathleen De Vera BarrilNoch keine Bewertungen
- LAB LLE FULL REPORT ZkinDokument26 SeitenLAB LLE FULL REPORT ZkinAmir Al-AimanNoch keine Bewertungen
- Understanding Reaction Kinetics in Batch and Continuous ReactorsDokument14 SeitenUnderstanding Reaction Kinetics in Batch and Continuous ReactorsAmy Farhana33% (3)
- Final Report PFRDokument12 SeitenFinal Report PFRmark_ancotNoch keine Bewertungen
- Cre Lab ManualsDokument18 SeitenCre Lab ManualsRishavKrishna100% (1)
- CSTR 40L LAB EXPERIMENTDokument18 SeitenCSTR 40L LAB EXPERIMENTSaber Minato Azrul100% (2)
- Tenses Table PDFDokument5 SeitenTenses Table PDFAlejandra Neira GonzálezNoch keine Bewertungen
- Pinch TechDokument32 SeitenPinch TechDaniel Puello Rodelo100% (1)
- Defluoridation ReportDokument62 SeitenDefluoridation ReportS M Aseem100% (1)
- Hindi Typing by English KeyboardDokument1 SeiteHindi Typing by English KeyboardS M AseemNoch keine Bewertungen
- CH Gate'14Dokument24 SeitenCH Gate'14richarai2312Noch keine Bewertungen
- 12 Chapter3 Section4 Oil Refining Industry Page193 220Dokument26 Seiten12 Chapter3 Section4 Oil Refining Industry Page193 220Muhammad FarooqNoch keine Bewertungen
- Defluoridation ReportDokument62 SeitenDefluoridation ReportS M Aseem100% (1)
- Quantitative Abilities: Centres atDokument12 SeitenQuantitative Abilities: Centres atS M AseemNoch keine Bewertungen
- Purification process flow diagram for cadmium recoveryDokument1 SeitePurification process flow diagram for cadmium recoveryAkash BansalNoch keine Bewertungen
- Chap 04Dokument24 SeitenChap 04S M AseemNoch keine Bewertungen
- Refining HistoryDokument26 SeitenRefining HistoryS M AseemNoch keine Bewertungen
- Weak Acid, Strong Base Titration Lab Chemistry 20 TEACHER NotesDokument3 SeitenWeak Acid, Strong Base Titration Lab Chemistry 20 TEACHER NotesArash JoonNoch keine Bewertungen
- Exeter Politics DissertationDokument6 SeitenExeter Politics DissertationWriteMyPaperForMeCheapNewHaven100% (1)
- Concept of The ResearchDokument42 SeitenConcept of The ResearchEthiopia NetsanetNoch keine Bewertungen
- Probability 1Dokument113 SeitenProbability 1GizawNoch keine Bewertungen
- Table of Contents Hand MoisturizerDokument3 SeitenTable of Contents Hand MoisturizerDavidNoch keine Bewertungen
- Masters in Education Dissertation ExamplesDokument7 SeitenMasters in Education Dissertation ExamplesBestOnlinePaperWritingServiceUK100% (1)
- The Parenting Style and The Academic Performance of Grade 6 Pupils of Saint Mary'S College of CatbaloganDokument24 SeitenThe Parenting Style and The Academic Performance of Grade 6 Pupils of Saint Mary'S College of CatbaloganVincent Nalazon-Caranog Pamplina-ArcallanaNoch keine Bewertungen
- F TestDokument7 SeitenF TestShamik MisraNoch keine Bewertungen
- Design Criteria For An Urban Sidewalk LandscapeDokument8 SeitenDesign Criteria For An Urban Sidewalk LandscapePaola PacchaNoch keine Bewertungen
- Ezekiel Project Work-1Dokument35 SeitenEzekiel Project Work-1saheedNoch keine Bewertungen
- SESI 5 Element of Research DesignDokument12 SeitenSESI 5 Element of Research DesignStevia Tjioe100% (1)
- Syllabus Business Analytics Second Sem Sy 2019-2020Dokument5 SeitenSyllabus Business Analytics Second Sem Sy 2019-2020Phia BrumaNoch keine Bewertungen
- 1 s2.0 S0003682X12001545 Main PDFDokument8 Seiten1 s2.0 S0003682X12001545 Main PDFCristóbal BriceñoNoch keine Bewertungen
- TQM in Auto IndustryDokument55 SeitenTQM in Auto IndustryPon VenkateshNoch keine Bewertungen
- Quantitative Research SIMDokument18 SeitenQuantitative Research SIMSandara YansonNoch keine Bewertungen
- Autoethnography and Family ResearchDokument18 SeitenAutoethnography and Family ResearchpecescdNoch keine Bewertungen
- Factors Affecting Short Term Memory - b2Dokument11 SeitenFactors Affecting Short Term Memory - b2Mohisha Vaartini SubramaniamNoch keine Bewertungen
- Birla Institute of Technology & Science, Pilani Work Integrated Learning Programmes Division Manufacturing ExcellenceDokument7 SeitenBirla Institute of Technology & Science, Pilani Work Integrated Learning Programmes Division Manufacturing Excellencebalaji817150Noch keine Bewertungen
- CFITDokument18 SeitenCFITKriti ShettyNoch keine Bewertungen
- 5.1 Mining Data StreamsDokument16 Seiten5.1 Mining Data StreamsRaj EndranNoch keine Bewertungen
- Criteria-Based Assessment and Grading in Architecture Design StudioDokument8 SeitenCriteria-Based Assessment and Grading in Architecture Design StudiolalecrimNoch keine Bewertungen
- Box Plot Answers MMEDokument2 SeitenBox Plot Answers MMESabih AzharNoch keine Bewertungen
- Final RM ProjectDokument17 SeitenFinal RM ProjectJignesh VasaniNoch keine Bewertungen
- Assignment #2 Confidence Interval EstimationDokument5 SeitenAssignment #2 Confidence Interval EstimationRania ChoucheneNoch keine Bewertungen
- BIRCH: Balanced Iterative Reducing and Clustering using HierarchiesDokument33 SeitenBIRCH: Balanced Iterative Reducing and Clustering using HierarchiesSpandan RoyNoch keine Bewertungen
- 301 2024Dokument8 Seiten301 2024Mandisa NgemaNoch keine Bewertungen
- API 580 QB - Mock Up Exam For 5 TH Day-NO KEYDokument25 SeitenAPI 580 QB - Mock Up Exam For 5 TH Day-NO KEYsebincherianNoch keine Bewertungen
- Performing a Paired Data Nonparametric TestDokument7 SeitenPerforming a Paired Data Nonparametric TestAzriNexusNoch keine Bewertungen
- Pa Turnpike Design Consistancy Manual 2011Dokument208 SeitenPa Turnpike Design Consistancy Manual 2011aapennsylvaniaNoch keine Bewertungen
- CC 22102015 CorrectionDokument4 SeitenCC 22102015 CorrectionAnne Marielle AmparadoNoch keine Bewertungen
- The Fabric of Civilization: How Textiles Made the WorldVon EverandThe Fabric of Civilization: How Textiles Made the WorldBewertung: 4.5 von 5 Sternen4.5/5 (57)
- Sully: The Untold Story Behind the Miracle on the HudsonVon EverandSully: The Untold Story Behind the Miracle on the HudsonBewertung: 4 von 5 Sternen4/5 (103)
- Dirt to Soil: One Family’s Journey into Regenerative AgricultureVon EverandDirt to Soil: One Family’s Journey into Regenerative AgricultureBewertung: 5 von 5 Sternen5/5 (124)
- Faster: How a Jewish Driver, an American Heiress, and a Legendary Car Beat Hitler's BestVon EverandFaster: How a Jewish Driver, an American Heiress, and a Legendary Car Beat Hitler's BestBewertung: 4 von 5 Sternen4/5 (28)
- Recording Unhinged: Creative and Unconventional Music Recording TechniquesVon EverandRecording Unhinged: Creative and Unconventional Music Recording TechniquesNoch keine Bewertungen
- Pale Blue Dot: A Vision of the Human Future in SpaceVon EverandPale Blue Dot: A Vision of the Human Future in SpaceBewertung: 4.5 von 5 Sternen4.5/5 (586)
- Highest Duty: My Search for What Really MattersVon EverandHighest Duty: My Search for What Really MattersNoch keine Bewertungen
- The Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaVon EverandThe Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaNoch keine Bewertungen
- The Weather Machine: A Journey Inside the ForecastVon EverandThe Weather Machine: A Journey Inside the ForecastBewertung: 3.5 von 5 Sternen3.5/5 (31)
- Transformed: Moving to the Product Operating ModelVon EverandTransformed: Moving to the Product Operating ModelBewertung: 4 von 5 Sternen4/5 (1)
- A Place of My Own: The Architecture of DaydreamsVon EverandA Place of My Own: The Architecture of DaydreamsBewertung: 4 von 5 Sternen4/5 (241)
- 35 Miles From Shore: The Ditching and Rescue of ALM Flight 980Von Everand35 Miles From Shore: The Ditching and Rescue of ALM Flight 980Bewertung: 4 von 5 Sternen4/5 (21)
- Across the Airless Wilds: The Lunar Rover and the Triumph of the Final Moon LandingsVon EverandAcross the Airless Wilds: The Lunar Rover and the Triumph of the Final Moon LandingsNoch keine Bewertungen
- Packing for Mars: The Curious Science of Life in the VoidVon EverandPacking for Mars: The Curious Science of Life in the VoidBewertung: 4 von 5 Sternen4/5 (1395)
- The Future of Geography: How the Competition in Space Will Change Our WorldVon EverandThe Future of Geography: How the Competition in Space Will Change Our WorldBewertung: 4.5 von 5 Sternen4.5/5 (4)
- The Technology Trap: Capital, Labor, and Power in the Age of AutomationVon EverandThe Technology Trap: Capital, Labor, and Power in the Age of AutomationBewertung: 4.5 von 5 Sternen4.5/5 (46)
- Einstein's Fridge: How the Difference Between Hot and Cold Explains the UniverseVon EverandEinstein's Fridge: How the Difference Between Hot and Cold Explains the UniverseBewertung: 4.5 von 5 Sternen4.5/5 (50)
- Data-ism: The Revolution Transforming Decision Making, Consumer Behavior, and Almost Everything ElseVon EverandData-ism: The Revolution Transforming Decision Making, Consumer Behavior, and Almost Everything ElseBewertung: 3.5 von 5 Sternen3.5/5 (12)
- Broken Money: Why Our Financial System is Failing Us and How We Can Make it BetterVon EverandBroken Money: Why Our Financial System is Failing Us and How We Can Make it BetterBewertung: 5 von 5 Sternen5/5 (3)
- A Garden of Marvels: How We Discovered that Flowers Have Sex, Leaves Eat Air, and Other Secrets of PlantsVon EverandA Garden of Marvels: How We Discovered that Flowers Have Sex, Leaves Eat Air, and Other Secrets of PlantsNoch keine Bewertungen
- Mental Math for Pilots: A Study GuideVon EverandMental Math for Pilots: A Study GuideBewertung: 0.5 von 5 Sternen0.5/5 (1)
- The End of Craving: Recovering the Lost Wisdom of Eating WellVon EverandThe End of Craving: Recovering the Lost Wisdom of Eating WellBewertung: 4.5 von 5 Sternen4.5/5 (80)
- ChatGPT Money Machine 2024 - The Ultimate Chatbot Cheat Sheet to Go From Clueless Noob to Prompt Prodigy Fast! Complete AI Beginner’s Course to Catch the GPT Gold Rush Before It Leaves You BehindVon EverandChatGPT Money Machine 2024 - The Ultimate Chatbot Cheat Sheet to Go From Clueless Noob to Prompt Prodigy Fast! Complete AI Beginner’s Course to Catch the GPT Gold Rush Before It Leaves You BehindNoch keine Bewertungen
- Artificial Intelligence: A Guide for Thinking HumansVon EverandArtificial Intelligence: A Guide for Thinking HumansBewertung: 4.5 von 5 Sternen4.5/5 (30)
- Reality+: Virtual Worlds and the Problems of PhilosophyVon EverandReality+: Virtual Worlds and the Problems of PhilosophyBewertung: 4 von 5 Sternen4/5 (24)