Beruflich Dokumente
Kultur Dokumente
IR Inquiry Experiment Objectives: Student Handout
Hochgeladen von
Nadia GonzalezOriginalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
IR Inquiry Experiment Objectives: Student Handout
Hochgeladen von
Nadia GonzalezCopyright:
Verfügbare Formate
Bennett & Forster, IR Cards.
Student Handout
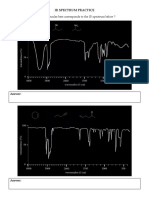
IR Inquiry Experiment Objectives to gain more practice recognizing functional groups from structures to gain practice drawing skeletal structures to figure out what IR peaks correspond to what bond types in molecules to learn to use IR spectra to recognize what functional groups are present in a sample
General tips and guidelines: 1. Work in groups of 2-3. Turn in one copy of the worksheet for your group. Make sure all partner names are included. 2. Check out a deck of IR cards 3. Do not write on the IR cards. Alphabetize and return them when you are finished. 4. Ignore any peaks below 1500 cm-1, unless they are particularly prominent. 5. A jagged peak in either direction just below 2400 cm-1 is from an incorrect background subtract of CO2. This peak arises from the constantly changing levels of CO2 in the room. 6. Some peaks may not be marked with numbers but you can estimate what their values are by using the scale on the x-axis. Do not assume that an unmarked peak is an unimportant peak. Some very important peaks are quite subtle (see examples on the next page). 7. All peaks above 1500 cm-1, aside from the CO2 peak, are important. Procedure 1. Sort through the stack of spectra cards, placing all examples of each functional group (FG) into separate piles. There should be 3-4 examples for each functional group. a. alkane only (none of the following FGs present) b. alkene only (none of the following FGs present) c. aromatic only (none of the following FGs present) d. alcohol (this FG and the subsequent ones may include the above FGs also) e. phenol f. carboxylic acid g. ketone h. aldehyde i. ester 2. Place all FG spectra in a vertical array and then try to find characteristics that they all share. When looking for IR peak characteristics you want to consider: a. location: the center of the peak in wavenumbers, cm-1; you may need to estimate some of these, broad peaks should be recorded as a range, e.g., 3500-3200 cm-1 b. intensity: how big/strong a peak is relative to the other peaks, y-axis: tallest peak = very strong, ~75% of tallest peak = strong, ~30-60% of tallest peak = moderate, <25% of tallest peak = weak) c. breadth: how wide a peak issharp <50 cm-1, moderate 50-150 cm-1, broad >150 cm-1) 3. Once you have filled in the worksheet and summarized your findings in your notebook, use this information as a reference to figure out the functional groups in your experimental IR spectra for subsequent labs. 4. Re-alphabetize the cards and return them.
S1
Bennett & Forster, IR Cards.
Examples:
CO2
CO2
S2
Bennett & Forster, IR Cards.
Student Worksheet IR Inquiry Experiment Due at the end of lab today Functional Groups: Provide the names and structures from the cards that have the following functional groups. All functional groups have 3-4 examples. alkane only
alkene only
aromatic only
alcohol
phenol
S3
Bennett & Forster, IR Cards.
carboxylic acid
ketone
aldehyde
ester
S4
Bennett & Forster, IR Cards.
Characteristics of functional groups in IR spectra: Fill in the following chart with up to three shared peaks that are characteristic of a functional group. Some locations are best reported as ranges. Functional Group e.g., carboxylic acid e.g., aldehyde Alkane Location ~2500-3500 ~2720-2750 Intensity Weak Very weak Breadth Very broad Moderate
Aromatic
Alkene
Alcohol
Phenol
Carboxylic acid
Ketone
Aldehyde
Ester
S5
Bennett & Forster, IR Cards.
Answer the following questions: 5. What effect does conjugation (alternating pi bonds) have on the location of C=O peaks? First find all examples of the C=O functional groups and then determine which peak they all share. Then compare that peak within a functional group between the conjugated and unconjugated examples to determine the effect of conjugation. Each C=O containing functional group has two conjugated examples and two unconjugated examples. Explain, using specific examples.
6. How can you distinguish an alkene from an aromatic using IR spectra? Explain, using specific examples.
7. How can you distinguish alcohols, phenols, and carboxylic acids from one another using IR spectra? Explain, using specific examples.
8. How can you distinguish an aldehyde from a ketone using IR spectra? Explain, using specific examples.
9. How can you distinguish a carboxylic acid from an ester using IR spectra? Explain, using specific examples.
S6
Das könnte Ihnen auch gefallen
- Cartas IR SI-editadoDokument21 SeitenCartas IR SI-editadoLuis Petrikowski :3Noch keine Bewertungen
- 1 - Lab Safety & IR SpectrosDokument6 Seiten1 - Lab Safety & IR Spectrosc88y45hbnqNoch keine Bewertungen
- 08 - Infrared Spectroscopy ManualDokument4 Seiten08 - Infrared Spectroscopy ManualIan RidzuanNoch keine Bewertungen
- 3-4 IR SpectrosDokument77 Seiten3-4 IR SpectrosRike AndrianiNoch keine Bewertungen
- IR Dry LabDokument17 SeitenIR Dry LabhimaNoch keine Bewertungen
- 4.11 Structure DeterminationDokument46 Seiten4.11 Structure DeterminationJaslinder Kaur Dhillon100% (1)
- Practical 1 Absorption SpectrosDokument11 SeitenPractical 1 Absorption SpectrosKjell CornelisNoch keine Bewertungen
- Chem 24 Pal Worksheet IrDokument4 SeitenChem 24 Pal Worksheet IrAmbreen latif Abdul latifNoch keine Bewertungen
- IR ProcedureDokument5 SeitenIR ProcedureMuhammad FauziNoch keine Bewertungen
- Spec Prob Set 315 CurrentDokument20 SeitenSpec Prob Set 315 CurrentUmang Agarwal57% (7)
- IR Spectroscopy LabDokument10 SeitenIR Spectroscopy LabChristian AmpeNoch keine Bewertungen
- Question 1. Which of These Molecules Best Corresponds To The IR Spectrum Below ?Dokument34 SeitenQuestion 1. Which of These Molecules Best Corresponds To The IR Spectrum Below ?Nguyễn ĐạtNoch keine Bewertungen
- 08 - Infrared Spectroscopy ManualDokument5 Seiten08 - Infrared Spectroscopy ManualShubham BobadeNoch keine Bewertungen
- Looking at Infrared Spectra:: ExampleDokument4 SeitenLooking at Infrared Spectra:: ExampleRamshaNoch keine Bewertungen
- Interpreting IRDokument5 SeitenInterpreting IRzebasiltNoch keine Bewertungen
- IR Spectra: Tricks For Identifying The 5 Zones: Why Is It Useful?Dokument7 SeitenIR Spectra: Tricks For Identifying The 5 Zones: Why Is It Useful?roNoch keine Bewertungen
- Tutorial62 PDFDokument7 SeitenTutorial62 PDFElMaharajaNoch keine Bewertungen
- EBatistil - Problem Set 1Dokument8 SeitenEBatistil - Problem Set 1essielveNoch keine Bewertungen
- Interpretation of IR Compounds BY KhanDokument7 SeitenInterpretation of IR Compounds BY KhanShamsheer KhanNoch keine Bewertungen
- Ir PDFDokument1 SeiteIr PDFBartłomiej LesiszNoch keine Bewertungen
- Experiment 7Dokument10 SeitenExperiment 7NathanianNoch keine Bewertungen
- CPR 1 IR SpectraDokument2 SeitenCPR 1 IR SpectrarunninkeysNoch keine Bewertungen
- IREDPPDokument30 SeitenIREDPPapi-3710134Noch keine Bewertungen
- Infrared Spectros PDFDokument33 SeitenInfrared Spectros PDFMuhammad BilalNoch keine Bewertungen
- Functional Groups Functional Groups: Functional Group G PDokument52 SeitenFunctional Groups Functional Groups: Functional Group G PZenonissya Galwan BataraNoch keine Bewertungen
- FTIRDokument8 SeitenFTIRsamrinaNoch keine Bewertungen
- 054 Infrared Spectro PDFDokument3 Seiten054 Infrared Spectro PDFThuvarakaNoch keine Bewertungen
- IR SpectraDokument5 SeitenIR SpectraXyphrus EmeritusNoch keine Bewertungen
- Infraredspectroscopy 161018121240Dokument33 SeitenInfraredspectroscopy 161018121240Rajkishor YadavNoch keine Bewertungen
- Infra Red SpectrosDokument30 SeitenInfra Red Spectrosboyi yangNoch keine Bewertungen
- Experiment 3 An Introduction To Functional Groups in Organic MoleculesDokument5 SeitenExperiment 3 An Introduction To Functional Groups in Organic MoleculesUnaka MaxmNoch keine Bewertungen
- Chemistry 263D: Organic Chemistry I Homework 9, Fall 2021 NameDokument6 SeitenChemistry 263D: Organic Chemistry I Homework 9, Fall 2021 NameAndy CookNoch keine Bewertungen
- Ir AssignmentDokument5 SeitenIr AssignmentafodbdskjhibkoNoch keine Bewertungen
- تشخيص من النتDokument6 Seitenتشخيص من النتbsmalah11alroxnamNoch keine Bewertungen
- CHM580-Tutorial IR Dan RamanDokument6 SeitenCHM580-Tutorial IR Dan RamanSuhailyShukri100% (1)
- Organic Chemistry For Aspiring Pharmacists PART 1Dokument95 SeitenOrganic Chemistry For Aspiring Pharmacists PART 1Jessica GutierrezNoch keine Bewertungen
- Virtual Experiment 2: Instrumentation and Working Principles of Infrared (IR) SpectrosDokument10 SeitenVirtual Experiment 2: Instrumentation and Working Principles of Infrared (IR) SpectrosBernadette Virola CuevasNoch keine Bewertungen
- IR Spectroscopy GeneralDokument3 SeitenIR Spectroscopy GeneralMunish AroraNoch keine Bewertungen
- Analysis of Aspirin - Infrared (Ir) Spectroscopy and Melting Point DeterminationDokument15 SeitenAnalysis of Aspirin - Infrared (Ir) Spectroscopy and Melting Point DeterminationMahmoud ElshahawyNoch keine Bewertungen
- FTIR - LablingDokument21 SeitenFTIR - LablingJihan AfifahNoch keine Bewertungen
- IR Spectroscopy 2chem 2022Dokument52 SeitenIR Spectroscopy 2chem 2022John Jeric San JoseNoch keine Bewertungen
- RUBY ExpDokument7 SeitenRUBY ExpWan AizuddinNoch keine Bewertungen
- INFRARED SPECTROSCOPY (Full Lecture)Dokument74 SeitenINFRARED SPECTROSCOPY (Full Lecture)Alexine CharlonNoch keine Bewertungen
- Interpreting of 1 HexeneDokument1 SeiteInterpreting of 1 HexeneShamsheer KhanNoch keine Bewertungen
- IR ProcedureDokument5 SeitenIR ProcedurePuvaneswary LoganathanNoch keine Bewertungen
- Infrared Spectrum Formal Lab ReportDokument10 SeitenInfrared Spectrum Formal Lab ReportAnonymous 1z7otENoch keine Bewertungen
- IR QualitativeDokument4 SeitenIR QualitativeredaelwanNoch keine Bewertungen
- CHM260 Ftir Lab ReportDokument6 SeitenCHM260 Ftir Lab ReportAIN NABILA SHAHARUDDINNoch keine Bewertungen
- Ftir SpectrosDokument36 SeitenFtir SpectrosHafiz Mudaser AhmadNoch keine Bewertungen
- EECS 100/43 Lab 4 - Linear Op-Amp Circuits: 1. ObjectiveDokument7 SeitenEECS 100/43 Lab 4 - Linear Op-Amp Circuits: 1. Objectivespidy_171Noch keine Bewertungen
- Xiao 101083582 IRDokument6 SeitenXiao 101083582 IRzhiqiaoxiao7Noch keine Bewertungen
- Infrared and Raman Spectroscopy: Principles and Spectral InterpretationVon EverandInfrared and Raman Spectroscopy: Principles and Spectral InterpretationBewertung: 3 von 5 Sternen3/5 (1)
- Ir PresentationDokument24 SeitenIr PresentationMuneebNoch keine Bewertungen
- Main Body and General InstructionsDokument11 SeitenMain Body and General InstructionsAreeba IshtiaqNoch keine Bewertungen
- Infrared Spectrosco PY: By: Sarthak PradhanDokument25 SeitenInfrared Spectrosco PY: By: Sarthak PradhanSarthak100% (1)
- Infrared Spectroscopy: Theory and Interpretation of IR SpectraDokument33 SeitenInfrared Spectroscopy: Theory and Interpretation of IR SpectraGerald See TohNoch keine Bewertungen
- Experiment 3 Fourier Transform Infrared Spectroscopy (FTIR)Dokument7 SeitenExperiment 3 Fourier Transform Infrared Spectroscopy (FTIR)Muhammad Azri HaziqNoch keine Bewertungen
- The Handbook of Infrared and Raman Characteristic Frequencies of Organic MoleculesVon EverandThe Handbook of Infrared and Raman Characteristic Frequencies of Organic MoleculesBewertung: 5 von 5 Sternen5/5 (2)
- Passive and Active RF-Microwave Circuits: Course and Exercises with SolutionsVon EverandPassive and Active RF-Microwave Circuits: Course and Exercises with SolutionsNoch keine Bewertungen
- HOMEWORK ASSIGNMENT 13: Solutions: Z Z 2 Z Z Z Z 2 Z Z Z Z Z 2 Z Z Z Z Z Z Z 2Dokument13 SeitenHOMEWORK ASSIGNMENT 13: Solutions: Z Z 2 Z Z Z Z 2 Z Z Z Z Z 2 Z Z Z Z Z Z Z 2Randeep Iyyad N CNoch keine Bewertungen
- Sylgard HVIC Plus PDSDokument4 SeitenSylgard HVIC Plus PDSProject Sales CorpNoch keine Bewertungen
- INTRO To ORGANIC CHEMISTRYDokument60 SeitenINTRO To ORGANIC CHEMISTRYNailah KaharNoch keine Bewertungen
- G484 Jan 11Dokument12 SeitenG484 Jan 11samy9387Noch keine Bewertungen
- POE City Owners Manual SXT 2011Dokument16 SeitenPOE City Owners Manual SXT 2011vaglohrdNoch keine Bewertungen
- FRP Ship RuleDokument103 SeitenFRP Ship Rulereza84Noch keine Bewertungen
- AN 280 IC Carbohydrates Coffee HPAE PAD AN70231 ENDokument12 SeitenAN 280 IC Carbohydrates Coffee HPAE PAD AN70231 ENjoann bNoch keine Bewertungen
- Des Case PML Manual DigitalDokument195 SeitenDes Case PML Manual DigitalFraz Ahmad0% (1)
- Spartan 16 ManualDokument579 SeitenSpartan 16 ManualwuNoch keine Bewertungen
- TreasuryDM1 2 CDokument4 SeitenTreasuryDM1 2 CbalasukNoch keine Bewertungen
- Aws D1.1 - WPS - Smaw-Fcaw PDFDokument1 SeiteAws D1.1 - WPS - Smaw-Fcaw PDFBernathTurnipNoch keine Bewertungen
- The 13 Most Important Numbers in The Universe - James D. Stein's Cosmic NumbersDokument10 SeitenThe 13 Most Important Numbers in The Universe - James D. Stein's Cosmic NumbersEmerson Novais OliveiraNoch keine Bewertungen
- Keragaan Nilai DO, BOD Dan COD Di Danau Bekas Tambang Batu BaraDokument8 SeitenKeragaan Nilai DO, BOD Dan COD Di Danau Bekas Tambang Batu Baranurul_595600924Noch keine Bewertungen
- What Is Atmospheric PressureDokument10 SeitenWhat Is Atmospheric Pressurenidyashree100% (1)
- Chem 16 Long Exam 1 ReviewerDokument4 SeitenChem 16 Long Exam 1 Reviewerdesperateboy100% (1)
- Introduction To Artificial Neural Network (ANN) Methods: What They Are and How To Use ThemDokument27 SeitenIntroduction To Artificial Neural Network (ANN) Methods: What They Are and How To Use ThemavinashpatilNoch keine Bewertungen
- Activity Sheets For Chem With NamesDokument6 SeitenActivity Sheets For Chem With Namesapi-283862617100% (1)
- Rheology of Drilling MudDokument20 SeitenRheology of Drilling MudAnonymous bFVPpQjwj0% (1)
- ACOUSTICS Enclosure Design OKDokument51 SeitenACOUSTICS Enclosure Design OKcatanino100% (1)
- Sikagard - 694 F (I) : Moisture Insensitive Epoxy PuttyDokument3 SeitenSikagard - 694 F (I) : Moisture Insensitive Epoxy Puttykartick adhikaryNoch keine Bewertungen
- Planck Constant - Wikipedia, The Free EncyclopediaDokument15 SeitenPlanck Constant - Wikipedia, The Free Encyclopediad_richard_dNoch keine Bewertungen
- Shotcrete Evaluation and TestingDokument5 SeitenShotcrete Evaluation and TestingMohammed ZaheriNoch keine Bewertungen
- Database 2Dokument2.539 SeitenDatabase 2Shreyas07100% (2)
- Hi9813 6 - Hi9813 5Dokument4 SeitenHi9813 6 - Hi9813 5Vani IINoch keine Bewertungen
- Easby 1966Dokument12 SeitenEasby 1966enxNoch keine Bewertungen
- Mouse Cell Surface MarkersDokument9 SeitenMouse Cell Surface Markersavalon784Noch keine Bewertungen
- Wastewater Reuse and Current ChallengesDokument271 SeitenWastewater Reuse and Current ChallengesAlexis Utrers100% (2)
- AP Practice - Chapter 8 & 9 - 10Dokument3 SeitenAP Practice - Chapter 8 & 9 - 10Harin ParikhNoch keine Bewertungen