Beruflich Dokumente
Kultur Dokumente
Estrification
Hochgeladen von
Syed Shah Jehan GillaniOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Estrification
Hochgeladen von
Syed Shah Jehan GillaniCopyright:
Verfügbare Formate
Esterification
It is the Process by which esters are produced. These are widely used in various industries. An ester is defined as a compound formed by substituting organic radicals for ionizable hydrogen of an acid. The mechanism by which this replacement occurs has been well established e.g.; when acetic acid is esterified with alcohol it is evident that either the carbonyl oxygen bond or the alkyl-oxygen will break. CH3COOH + CH3 CH2OH CH3 CO.OC2H5 + H2O
Evidence for breaking of carbonyl-oxygen bond was found in the study of the following reaction in which water formed. CH3COOH + CH3CH2SH CH3 CO.SC2H5 + H2O
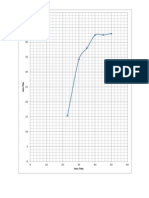
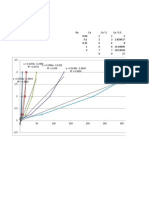
If the alkyl-Sulphur bond had broken H2S would have been formed. It will be observed that of all the alcohols studied, methyl alcohol shows the greatest initial rate of esterification and the highest limit. The primary alcohols like ethyl, propyl and butyl have approximated the same initial rates and limits but inferior to methyl alcohol in both of these respects. It may be stated generally that the more branched the carbon chain of alcohol and the nearer the group, the slower will be the esterification.
O Ch3 C OH
CATALYTIC ESTRIFICATION
It samples are taken at intervals from a mixture of acetic acid and the ethyl alcohol at room temperature and treated, a slow decrease in acidity can be observed but even after very long time before the minimum value(or limit) is reached like most of the other reactions, the speed of esterification can be increased with temperature e.g. if the temperature is raised by 10 0C, the rate of esterification approximately doubles. Hence heat speeds up the esterification process. However in most instances heating alone does not speed up esterification to a practical rate. In the case of high boiling alcohols, like glycerol, with a high boiling acid such as stearic acid, esterification cant be affected at atmospheric pressure in a reasonable time without the use of catalyst. It is generally observed that process of esterification can be enormously hastened by addition of strong acid like H2SO4 or HCL . The equilibrium point is not changed with the addition of catalyst but only the rate of esterification is increased. Esterification proceeds by attack of an alcohol molecule on the slightly positive carbonyl carbon of an acid. The larger this positive charge, the more rapid the reaction will be. While the nature of alkyl group attached to a carbonyl group will influence this charge, methods . catalysts can be used to increase the positive charge so that a given acid will estrify more rapidly. Esterification catalysts are compounds which are acidic in nature and the acidic compounds are those in which the central atom has incomplete external electron shell. In this way, addition to
hydrogen ion H+ compounds, such as boron trifloride(BF3), aluminium chloride(ZnCl2) can be considered to be acids. The neutralization reaction of such an acid is written as the donation of an electron pair by the base to acid.
In certain cases when the acid is sufficiently strong the esterification is self catalysed the speed of the reaction can be increased also by working under increased pressure so that higher temperature can be used. Generally, H2SO4 and HCL are used as catalysts. H2SO4 may cause dehydration of an alcohol if used in too great amount or at a very high temperature. Thus carbohexanol when boiled for 30 minutes with two moles of acetic acid and 3% by volume of H2SO4 gives 30% cyclohexene/ The use of any strong acid as catalyst may cause isomerization or destruction of a tertiary alcohol such as linaloal. Perchloric and phosphoric acids have been recommended as catalysts. Phosphoric acid is less efficient but also less destructive. The sulphuric acid acids particularly hose containing a considerable number of carbon atoms are desireable catalysts on account of their higher efficiency, solubility in the higher alcohols and acids, and less destructive action. The most commonly used is ptoluenesulphonic acid. Ion exchange resins are excellent catalysts and they offer the advantage of being easily removed by filteration. Boron and siliconfluorides are also used as catalysts. Among metals, Ag, Bi, Cu, Zn, tin are considered active catalysts in finely divided state various patents claim the use of aluminium, cobalt, lead, magnesium, tin and zinc soaps as esterification catalysts.
COMLETING ESTERIFICATION
Various esterification methods, in which the equilibrium is displaced by removal of one of the reaction products, have been designed so that the ester is obtained in high yield and conversion. One way of completing esterification is to remove the water as it is formed. When the acids alcohol and ester are non-volatile, the mixture is heated usually to 200 0C (or higher) without catalyst to remove H2O. The removal of water is aided by bubbling an inert gas through the mixture or by application of a vacuum. Glycerides of nonvolatile acids are made in this way to ensure complete esterification of the trivalent glycerol, an excess of acid is used. This is later removed by an alkali wash. Ester gum, a widely used resin is esterified completely by heating glycerol with resin so as to drive out the water. Another method of removing water is to pass superheated steamthrough the mixture which agitates the mixture as well as driving off the water as it is formed.
When ether or both the acid and the alcohol are volatile, the reaction may be carried to completion by distilling out the water produced in the reaction, usually as an azeo-trope. The azeotrope can be water with one of the components of the reaction mixture or will an inert solvent which is insoluble in water. Generally an azeotrope is selected which has boiling point below 100 0C. In addition to the B.P of the azeotrope, the solubilities of the esters and alcohols in water must also be considered. The apparatus used for azeotropic esterification includes a receiver to which the two phases separated/ Generally ethyl alcohol is produced on this basis. The non aqueous phase automatically returns to esterification vessel by an overflow, while the aqueous phase is withdrawn from the bottom of the receiver.
Catalysts USED IN ESTERIFICATION
Strong HCL, H2SO4, BF3, AlCl3,ZnCl2, Perchloric acid, phosphoric acid, Sulphonic acid, ion exchange resins, silicon chloride. Among metals; Ag, bi, Cu, Tinchloride. Soaps of : Al,Co,Pb,Mg,Zn,and tin. Oxides of : Al,Pb,Mg.
Esterification of Carboxylic acid Derivatives.
ALCOHOLYSIS: In alcoholysis,,an alcohol reacts with an ester to give a new ester. CH3COO.C2H5 + HOCH3 CH3COOCH3 + HOC2H5
At room temperature in absence of a catalyst, equilibrium is established extremely slowly. The strong acids that are used as catalysts in ordinary esterification serve equally well for alcoholysis. But the most commonly used catalysts for alcoholysis are Sodiumalkoxides. These must be used in anyhydrous systems since they are hydrolyzed with water and the resulting hydroxides, hydrolyze the esters.The usual practice is to dissolve a small amount of sodium in the alcohol to be used and then added to the ester. Low concentration of these catalysts cause the transformation to take place rapidly even at room temperature. Sodium ehoxide is about one thousand times as active as an equivalent amount of HCL. An explanation for this increase in rate is that the basicity of the alcohol oxygen is increased and that this increase in basicity facilitates attack on the positive carbonyl carbon. In addition to sodium alkoxides, various other catalysts may be used such as NH3, Pyridine, NaOH,Tetra methyl ammonium hydroxide, aluminium alkoxides, lithium methoxide and Na2CO3.
Completing ALCOHOLYSIS Since alcoholysis is an uquilibrium reaction, the reaction must be forced to completion as in the case
of esterification. For this purpose, removal of the one of the reaction products permits the reaction to go to completion. Thus when higher alcohol such as butyl,amyl, or benzyl alcohol are heated under a fractionating coloumn with methyl or ethl acetate and a catalyst, the most volatile constituent methyl is left. Or the new ester may be distilled out as when ethanol reacts with glycol acetate. When the a glyceride is alcoholyzed by an alcohol such as C2H5OH, the glycerol seperates out due to its low solubility in esters and the reaction goes forwards completion and alcoholysis is repeated if necessary. This is practicable way of obtaining glycerol.
UTILIZATION OF ALCOHOLYSIS
Alcoholysis is used in the manufacturing of monomeric cyclic polymethylene carbonates which can be obtained in no other way. A higher polymethylene glycols and sodium are heated with butyle carbonate to obtain the polymeric polymethylene carbonate. The crude product, which still contains the catalyst, is heated in a high vacuum, the trace of monomeric cyclic ester present distills out and more is formed by rearrangement until nearly all the material is obtained in the desired form. The alcoholysis has recently been tried with glyceride s also. When a mixture of glyceride is heated with a catalyst they are inter-esterified. In some cases the drying properties of a mixture of oils are also much improved by this process. If the alcoholysis of a glyceride containing both saturated and unsaturated acids is effected below the melting points of more saturated , less soluable glycerides so that they separate out and the equilibrium will be destroyed and additional amounts of saturated glycerides will be formed.
EQUIPMENT AND OPERATION FOR ALCOHOLYSIS
The alcoholysis process uses anyhydrous esters, alcohols and sodium catalysts, none of which causes corrosion. The equipment may be quite simple and consist of a Kettle and coloumn with suitable attachments. Usually thealcohol is put in kettle first and the sodium metal(0.2-1.0 %) of the weight of ester os then introduced after the ester has been run in the volatile component is begun. The alcoholysis component process is also readily adaptable to continuous operation. Procedures in general are similar to those used for continuous esterification.
ACIDOLYSIS
An acid is reacted with an ester to produce new ester CH3COOC2H5 + C15H31COOH C15H31COOC2H5 + CH3COOH
The counterpart of alcoholysis is acidolysis , in which one acid displaces another from an ester. The alkaline catalysts which are so efficient in alcoholysis can be used, the slower acid catalysts or heat alone must be used. Boron tri fluoride is effective. Mercuru salts are recommended as catalysts for the acidolysis of vinyl esters. Quantiative studies show that one acid displaces another to about the extent that would be expected from esterification constants. The reaction goes to completion if the displaced acid can be eliminated . Volatile acids can be distilled out, either alone or will azeotropes.
Esterification BY ACID ANYHYDRIDES 1. RXn 2. Rxn 3. Rxn Reaction 1 to 3 go to completion because the product formed do not interact to produce the starting materials. Esterification of an alcohol by an anyhydride is more rapid than by carrespondinga acid. However, the relative velocities of the reaction of acetic anyhydride with different alcohols are much the same as the relative velocities of the reaction of acetic acid with there alcohols.
ESTERIFICATION FROM METAL SALTS OF ACID AND ALKYLHALIDES
When the metal salt of an acid is heated with alkyl halides, the corresponding alkyl ester is formed. CH3COONa + BrC2H5 CH3COOC2H5 + NaBr CH3COONa + ClCH2C6H5 CH3COOCH2C6H5 + NaCl
This type of reaction is used for preparing esters especially those which may be used for identification of the acid. Silve sals which are readily prepared from acids are frequently employed for this purpose because they do not require any solvent and the resulting ester needs little purification
Esters from nitriles
If the ester of an acid corresponding to an available nitrile is desired, it may be prepared by sapanifying the nitrile and then esterifying the acid in usual way.However ,one operation is saved if the ester is prepared directly from the nitrile the nitriles of hydroxyl acids in particular are readily obtained by the addition of hydrocyanic acid to an aldehyde. The hydroxyl nitrile is readily dehydrated to acrylonitrile, from which acrylic eslers generally are manufactured by alcoholysis. The esterification of the nitriles present no great difficulty. An amount of acid catalyst greater than that required to combine with ammonia that is formed must be used. Higher reaction temperatures and longer reaction times are required than for simple esterification. A common procedure is to dissolve the nitrile in the appropriate alcohol and to saturate resulting solution with HCl.
ESERS FROM ACCETYLENE
When C2H2 and CH3COOH are brought together with suitable catalyst a vinyl ester or an ester of ethylidene glycol is formed according to the reaction. 1, 2.
By altering the reaction condition, either of these two products are obtained. Vinyl acetate which is used for making polymers and ethylidene diacetate, which is used intermediate for the manufacturing of acetic anhydride, are produced on a large scale by these reactions. The reaction may be employed to other carboxylic acid and acetylene derivative. RXN On some cases strong acids like H2SO4 and phosphoric acid are used as catalyst. However they are used in conjuction with mercury salts. Other catalysts which have been discovered for the reaction include boron trifluoride (BF3) and the salts of various metals such as zinc silicate, zinc acetate and mercuric phosphate the addition may be affected in vapour phase over a solid catalyst at 200 0C or above.
ESTERS FROM CARBON MONOOXIDE
CO reacts with alcohol at an elevated temperature and high pressure in the presence of a metal alkoxide as catalyst to give an alkyl formate. CH3OH + CO HCOOCH3 In the presence of acids or boron trifluoride at somewhat higher temperature under high pressure, the product is an acid. The acid so formed may react with a second molecule of the alcohol so that final product is an ester. A single molecule of an ether may react with CO to
Das könnte Ihnen auch gefallen
- IBFT - Member Bank Account Number FormatDokument1 SeiteIBFT - Member Bank Account Number FormatSyed Shah Jehan GillaniNoch keine Bewertungen
- Computational and Experimental Modeling of Three-Phase Slurry-Bubble Column ReactorDokument72 SeitenComputational and Experimental Modeling of Three-Phase Slurry-Bubble Column ReactorSyed Shah Jehan GillaniNoch keine Bewertungen
- Application Longterm NewDokument4 SeitenApplication Longterm NewSyed Shah Jehan GillaniNoch keine Bewertungen
- Ffoorr Tthhee PpeeoopplleeDokument8 SeitenFfoorr Tthhee PpeeoopplleeSyed Shah Jehan GillaniNoch keine Bewertungen
- Four Factor Formula FinalDokument37 SeitenFour Factor Formula FinalSyed Shah Jehan GillaniNoch keine Bewertungen
- Faisal KhanDokument10 SeitenFaisal KhanSyed Shah Jehan GillaniNoch keine Bewertungen
- Carbon Dioxide Capture and Storage in The Nitrogen and SynGas Industries 2Dokument10 SeitenCarbon Dioxide Capture and Storage in The Nitrogen and SynGas Industries 2Syed Shah Jehan GillaniNoch keine Bewertungen
- 1990 Ifa Venice DybkjaerDokument25 Seiten1990 Ifa Venice DybkjaerSyed Shah Jehan GillaniNoch keine Bewertungen
- 97 6 TocDokument10 Seiten97 6 TocJose Luis Gutierrez MadariagaNoch keine Bewertungen
- Social Problems of AdolescentsDokument7 SeitenSocial Problems of AdolescentsSyed Shah Jehan GillaniNoch keine Bewertungen
- C.R.E Copy ChartDokument5 SeitenC.R.E Copy ChartSyed Shah Jehan GillaniNoch keine Bewertungen
- Social Problems of AdolescentsDokument22 SeitenSocial Problems of AdolescentsSyed Shah Jehan Gillani100% (1)
- CO2 Removal From SyngasDokument10 SeitenCO2 Removal From SyngasSyed Shah Jehan GillaniNoch keine Bewertungen
- University of Engineering & Technology, Lahore: Unofficial TranscriptDokument1 SeiteUniversity of Engineering & Technology, Lahore: Unofficial TranscriptSyed Shah Jehan GillaniNoch keine Bewertungen
- Book 1Dokument3 SeitenBook 1Syed Shah Jehan GillaniNoch keine Bewertungen
- Mass TransferDokument2 SeitenMass TransferSyed Shah Jehan GillaniNoch keine Bewertungen
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5795)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (74)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (400)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (121)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- Main CatalogueDokument12 SeitenMain Catalogueferpa_ferNoch keine Bewertungen
- Idioms and PharsesDokument0 SeitenIdioms and PharsesPratik Ramesh Pappali100% (1)
- Defects Lamellar TearingDokument6 SeitenDefects Lamellar Tearingguru_terexNoch keine Bewertungen
- PL00002949Dokument5 SeitenPL00002949Nino AlicNoch keine Bewertungen
- Withania DSC PDFDokument6 SeitenWithania DSC PDFEfsha KhanNoch keine Bewertungen
- EBANX Beyond Borders 2020Dokument71 SeitenEBANX Beyond Borders 2020Fernanda MelloNoch keine Bewertungen
- The Way Out of Alcoholism by Jack BolandDokument38 SeitenThe Way Out of Alcoholism by Jack BolandIma AardvarkNoch keine Bewertungen
- Evidence Based DesignDokument4 SeitenEvidence Based Designmartinadam82Noch keine Bewertungen
- CatalogDokument12 SeitenCatalogjonz afashNoch keine Bewertungen
- Preservative MaterialsDokument2 SeitenPreservative MaterialsmtcengineeringNoch keine Bewertungen
- Impact of Job Design On Employee Engagement: A Theoretical and Literature ReviewDokument6 SeitenImpact of Job Design On Employee Engagement: A Theoretical and Literature ReviewAnonymous CwJeBCAXpNoch keine Bewertungen
- Distilled Witch Hazel AVF-SP DWH0003 - June13 - 0Dokument1 SeiteDistilled Witch Hazel AVF-SP DWH0003 - June13 - 0RnD Roi SuryaNoch keine Bewertungen
- Transmission Lines SMART EDGE VILLARUEL For April 2024 v1Dokument89 SeitenTransmission Lines SMART EDGE VILLARUEL For April 2024 v1mayandichoso24Noch keine Bewertungen
- Transformers: Z Z Z S S Z S SDokument17 SeitenTransformers: Z Z Z S S Z S SSreenivasaraoDharmavarapu100% (1)
- Ce Mark - Application FormDokument3 SeitenCe Mark - Application Formrajivsinghal90Noch keine Bewertungen
- 8953-Specifications For Doosan Man 9l21 31Dokument7 Seiten8953-Specifications For Doosan Man 9l21 31Bae Juyeon100% (1)
- RNW Position PaperDokument2 SeitenRNW Position PaperGeraldene AcebedoNoch keine Bewertungen
- Design and Details of Elevated Steel Tank PDFDokument10 SeitenDesign and Details of Elevated Steel Tank PDFandysupaNoch keine Bewertungen
- 2016 Uptown Parksuites D3 PDFDokument54 Seiten2016 Uptown Parksuites D3 PDFArafat Domado100% (1)
- Class 7 Work Book Answers Acid Bases and SaltsDokument2 SeitenClass 7 Work Book Answers Acid Bases and SaltsGaurav SethiNoch keine Bewertungen
- Docu Ifps Users Manual LatestDokument488 SeitenDocu Ifps Users Manual LatestLazar IvkovicNoch keine Bewertungen
- Cultivation: Bellis Perennis Is A CommonDokument2 SeitenCultivation: Bellis Perennis Is A CommonpriyankaNoch keine Bewertungen
- Chapter 1 Fundamentals of Taxation by Cruz, Deschamps, Miswander, Prendergast, Schisler, and TroneDokument25 SeitenChapter 1 Fundamentals of Taxation by Cruz, Deschamps, Miswander, Prendergast, Schisler, and TroneReese Parker100% (4)
- W01 M58 6984Dokument30 SeitenW01 M58 6984MROstop.comNoch keine Bewertungen
- Uppercut MagazineDokument12 SeitenUppercut MagazineChris Finn100% (1)
- Proposal Semister ProjectDokument7 SeitenProposal Semister ProjectMuket AgmasNoch keine Bewertungen
- Governance StructureDokument1 SeiteGovernance StructureJoydip MukhopadhyayNoch keine Bewertungen
- Sithpat006ccc019 A - 2021.1Dokument34 SeitenSithpat006ccc019 A - 2021.1Mark Andrew Clarete100% (2)
- Musa Paradisiaca L. and Musa Sapientum L.: A Phytochemical and Pharmacological ReviewDokument8 SeitenMusa Paradisiaca L. and Musa Sapientum L.: A Phytochemical and Pharmacological ReviewDeviNoch keine Bewertungen
- Top 6 Beginner Work Out MistakesDokument4 SeitenTop 6 Beginner Work Out MistakesMARYAM GULNoch keine Bewertungen