Beruflich Dokumente
Kultur Dokumente
Brownian Poster Final
Hochgeladen von
becheanu13Originalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Brownian Poster Final
Hochgeladen von
becheanu13Copyright:
Verfügbare Formate
Einsteins Theory of Brownian Motion and Diffusion
What is Brownian Motion? In 1827, Robert Brown, an English botanist, observed under a microscope that pollen grains in water were in a constant state of agitation. He first thought that there might be something alive, but proved that was not so by observing the same kind of motion in inclusions in quartz that were millions of years old. He never was able to explain the observations In 1905, Albert Einstein published a theory that turned out through measurements by Jean Perrin to explain Browns observations. Here is the title and start of the paper: On the movement of small particles suspended in a stationary liquid demanded by the molecular-kinetic theory of heat In this paper it will be shown that, according to the molecularkinetic theory of heat, bodies of a microscopically visible size suspended in liquids must, as a result of thermal molecular motions, perform motions of such magnitudes that they can be easily observed with a microscope. It is possible that the motions to be discussed here are identical with so-called Brownian molecular motion; however, the data available to me on the latter are so imprecise that I could not form a judgment on the question.
Albert Einstein
Robert Brown
Einsteins statement that thermal molecular motions should be easily observed under a microscope stimulated Jean Perrin to make quantitative measurements, culminating in his book The Atoms in 1909.
"I did not believe that it was possible to study the Brownian motion with such a precision." Letter from Albert Einstein to Jean Perrin (1909).
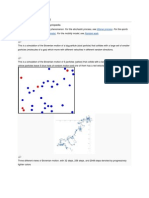
Einsteins explanation: The pollen grains are buffeted by collisions with molecules of water moving randomly in all directions (see figure above). Einsteins theory and Perrins observations showed that the mean distance traveled by a pollen grain (or other microscopic object) subject to random collisions increases as the square root of time:
, unlike the relation x ~ t for an object in straight-line motion.
Jean Perrin Drawings by Perrin of positions of a particle every 10 seconds, showing stochastic motion due to collisions with water molecules. Perrin was awarded the Nobel Prize in Physics in 1926
x~ t
Significance: The concept of atoms and molecules became universally accepted. Perrin deduced an accurate value for Avogadros number (the number of atoms in a mole). The theory applies to all diffusion phenomena: mixing of gases or liquids, atom motion in solids, spread of the black plague, spread of agriculture in Neolithic times, spread of clothing fashions, spread of africanized-bees.
Gary S. Collins, February 2005
Das könnte Ihnen auch gefallen
- Oxford Six Class Science NotesDokument15 SeitenOxford Six Class Science NotesLisha92% (12)
- 1957 HOUSNER Dynamic Pressures On Accelerated Fluid ContainersDokument21 Seiten1957 HOUSNER Dynamic Pressures On Accelerated Fluid ContainersEugenio Durban100% (1)
- Albert Einstein - Was He A Thief, A Liar and A PlagiaristDokument14 SeitenAlbert Einstein - Was He A Thief, A Liar and A Plagiaristknonie0% (2)
- Warm-Up Reading 1Dokument9 SeitenWarm-Up Reading 1benihimebankai92Noch keine Bewertungen
- Brownian MotionDokument31 SeitenBrownian Motionsujatha ramkiNoch keine Bewertungen
- Brownian Motion: From Wikipedia, The Free EncyclopediaDokument21 SeitenBrownian Motion: From Wikipedia, The Free EncyclopediarayroyNoch keine Bewertungen
- Karthikeya XII-A Physics Project PDFDokument23 SeitenKarthikeya XII-A Physics Project PDFkarthikeya kakarlapudiNoch keine Bewertungen
- Brownian Motion The Random Motion ofDokument1 SeiteBrownian Motion The Random Motion ofSujay BhattacharyaNoch keine Bewertungen
- Fatematun Nur Tofa (20201128)Dokument1 SeiteFatematun Nur Tofa (20201128)Ariful Hassan SaikatNoch keine Bewertungen
- Brownian MotionDokument25 SeitenBrownian Motionsujatha ramkiNoch keine Bewertungen
- BrownianDokument15 SeitenBrownianDeepak Prakash JayaNoch keine Bewertungen
- Brownian MotionDokument76 SeitenBrownian MotionPraveen KumarNoch keine Bewertungen
- Young EinsteinDokument10 SeitenYoung EinsteinkaubreyNoch keine Bewertungen
- A Ether DetectionDokument164 SeitenA Ether Detectionquicksilver2000Noch keine Bewertungen
- Vedanta and Quantum PhysicsDokument5 SeitenVedanta and Quantum Physicssudhakar_sudha2003Noch keine Bewertungen
- Eduardo V. Flores - Ether and The Theory of RelativityDokument2 SeitenEduardo V. Flores - Ether and The Theory of RelativityOppekeeNoch keine Bewertungen
- Albert Einsteinn - FinalDokument4 SeitenAlbert Einsteinn - FinalSharad PyakurelNoch keine Bewertungen
- From Einstein's Theorem To Bell's Theorem: A History of Quantum NonlocalityDokument18 SeitenFrom Einstein's Theorem To Bell's Theorem: A History of Quantum NonlocalityAnonymous IgWFxrNoch keine Bewertungen
- Phil EnidteDokument32 SeitenPhil EnidtegeniNoch keine Bewertungen
- A Brief History of Relativity - Stephan Hawkings 1999Dokument6 SeitenA Brief History of Relativity - Stephan Hawkings 1999TNT1842Noch keine Bewertungen
- 0063 0078Dokument16 Seiten0063 0078Anurag AnandNoch keine Bewertungen
- Ludwik Kostro - Albert Einstein's New Ether and His General RelativityDokument9 SeitenLudwik Kostro - Albert Einstein's New Ether and His General RelativityOppekeeNoch keine Bewertungen
- Einstein's Miraculous Argument of 1905 - The Thermodynamic Grounding of Light Quanta - 16Dokument16 SeitenEinstein's Miraculous Argument of 1905 - The Thermodynamic Grounding of Light Quanta - 16John BirdNoch keine Bewertungen
- 2017/3/6 ӥ܌11) 07 Einstein as a Jew and a Philosopher - by Freeman Dyson - The New York Review of BooksDokument2 Seiten2017/3/6 ӥ܌11) 07 Einstein as a Jew and a Philosopher - by Freeman Dyson - The New York Review of BooksCherryNoch keine Bewertungen
- Poincare's Ether ADokument13 SeitenPoincare's Ether ASadegh SimorghNoch keine Bewertungen
- Eddington-Einstein Eclipse 1919Dokument21 SeitenEddington-Einstein Eclipse 1919Lublinskaya100% (1)
- The Mystery of MatterDokument86 SeitenThe Mystery of MattercumdjafNoch keine Bewertungen
- Bilingual PlanDokument4 SeitenBilingual Planrenso bohorquez ramirezNoch keine Bewertungen
- Discoveries 1Dokument4 SeitenDiscoveries 1rkmgeciansandip1903Noch keine Bewertungen
- Sir Isac Newton - BiographyDokument12 SeitenSir Isac Newton - BiographyscoutjohnyNoch keine Bewertungen
- Einstein's Miraculous Argument of 1905: The Thermodynamic Grounding of Light QuantaDokument22 SeitenEinstein's Miraculous Argument of 1905: The Thermodynamic Grounding of Light QuantaMCarbajalNoch keine Bewertungen
- Uncertainty Einstein, Heisenberg, Bohr, and The Struggle For The Soul of Science by David Lindley Richard A Blasband (Vol 18 No 2)Dokument10 SeitenUncertainty Einstein, Heisenberg, Bohr, and The Struggle For The Soul of Science by David Lindley Richard A Blasband (Vol 18 No 2)Cambiador de MundoNoch keine Bewertungen
- The Einstein Theory of RelativityDokument34 SeitenThe Einstein Theory of RelativityStelioPassarisNoch keine Bewertungen
- Wave Particle DualityDokument7 SeitenWave Particle DualityRahmatullahNoch keine Bewertungen
- ChemistryDokument16 SeitenChemistrynehavj664Noch keine Bewertungen
- Annus Mirabilis Papers - WikipediaDokument47 SeitenAnnus Mirabilis Papers - Wikipediaminsara madtNoch keine Bewertungen
- Entropic StoryDokument19 SeitenEntropic StoryAlex ShoshitaishviliNoch keine Bewertungen
- The Albert Einstein Collection Volume Two: Essays in Science, Letters to Solovine, and Letters on Wave MechanicsVon EverandThe Albert Einstein Collection Volume Two: Essays in Science, Letters to Solovine, and Letters on Wave MechanicsNoch keine Bewertungen
- Secrets of Genius: Pergamon PressDokument14 SeitenSecrets of Genius: Pergamon Presssam kingNoch keine Bewertungen
- The Philosophy of Quantum TheoryDokument11 SeitenThe Philosophy of Quantum TheorySumbul ZehraNoch keine Bewertungen
- 404 737 1 SMDokument10 Seiten404 737 1 SMBishtNoch keine Bewertungen
- Einstein UploadDokument6 SeitenEinstein UploadMark AllenNoch keine Bewertungen
- Chasing The Light Einstein S Most Famous Thought Experiment: Thought Experiments in Philosophy, Science and The ArtsDokument22 SeitenChasing The Light Einstein S Most Famous Thought Experiment: Thought Experiments in Philosophy, Science and The ArtsStu DentNoch keine Bewertungen
- The Quantum Leap - FINAL PDFDokument13 SeitenThe Quantum Leap - FINAL PDFMuhammad Fahim HasanNoch keine Bewertungen
- Dragon Dreaming - Fact Sheet 1Dokument17 SeitenDragon Dreaming - Fact Sheet 1ningstriNoch keine Bewertungen
- Isaac Newton: BiographyDokument4 SeitenIsaac Newton: BiographychelseyNoch keine Bewertungen
- Brief Overview of TorsionDokument9 SeitenBrief Overview of TorsionGustavo Gabriel CiaNoch keine Bewertungen
- From Newton To Einstein: The Origins of General RelativityDokument5 SeitenFrom Newton To Einstein: The Origins of General RelativityHammad MisbahNoch keine Bewertungen
- Tombe - Einstein Big MistakeDokument10 SeitenTombe - Einstein Big Mistakerr100% (1)
- English Writing SampleDokument2 SeitenEnglish Writing SampleSouvik GhoshNoch keine Bewertungen
- UntitledDokument7 SeitenUntitledНазар МаксимюкNoch keine Bewertungen
- Galileo GalileiDokument7 SeitenGalileo GalileiTricia RuizNoch keine Bewertungen
- Essay Einsteins TimeDokument15 SeitenEssay Einsteins TimeRavi KapurNoch keine Bewertungen
- A New Quantum PhysicsDokument8 SeitenA New Quantum PhysicsPraveen PoddarNoch keine Bewertungen
- Reaction PapersDokument6 SeitenReaction PapersGrace SamNoch keine Bewertungen
- Einstein Theory of RelativityDokument26 SeitenEinstein Theory of RelativityPrince JurynNoch keine Bewertungen
- Physics in 17th CenturyDokument17 SeitenPhysics in 17th CenturyFrancis CorrosNoch keine Bewertungen
- The Einstein Theory of Relativity A Concise StatementVon EverandThe Einstein Theory of Relativity A Concise StatementBewertung: 3.5 von 5 Sternen3.5/5 (38)
- The Great Einstein Relativity Hoax and Other Science Questions, Hypotheses, and ImprobabilitiesVon EverandThe Great Einstein Relativity Hoax and Other Science Questions, Hypotheses, and ImprobabilitiesNoch keine Bewertungen
- Agitan DF 6120 Tds enDokument1 SeiteAgitan DF 6120 Tds enViktor Ragozin (Nakilon)Noch keine Bewertungen
- Seismic Design EC8Dokument15 SeitenSeismic Design EC8Wendirad Beshada100% (1)
- Why Does Charge Concentrate On Points?Dokument5 SeitenWhy Does Charge Concentrate On Points?Tashi DendupNoch keine Bewertungen
- B.T. Kumaon Institute of Technology, Dwarahat End Semester (Back) Examination, 2020-2021Dokument2 SeitenB.T. Kumaon Institute of Technology, Dwarahat End Semester (Back) Examination, 2020-2021verma.ashok031Noch keine Bewertungen
- Evaluation of Abelmoschus Moschatus Extracts For Antioxidant, Free Radical Scavenging, Antimicrobial and Antiproliferative Activities Using in Vitro AssaysDokument32 SeitenEvaluation of Abelmoschus Moschatus Extracts For Antioxidant, Free Radical Scavenging, Antimicrobial and Antiproliferative Activities Using in Vitro AssaysPrineteejayNoch keine Bewertungen
- 9800 XRF XRD - ArtigoDokument8 Seiten9800 XRF XRD - Artigocelestino biasottoNoch keine Bewertungen
- The Solubility of Gases in LiquidsDokument86 SeitenThe Solubility of Gases in Liquidsga6ba5Noch keine Bewertungen
- Cyanobacteria: A Precious Bio-Resource in Agriculture, Ecosystem, and Environmental SustainabilityDokument6 SeitenCyanobacteria: A Precious Bio-Resource in Agriculture, Ecosystem, and Environmental SustainabilityJoshua De LeonNoch keine Bewertungen
- Cambridge International AS & A Level: Chemistry 9701/42 October/November 2020Dokument14 SeitenCambridge International AS & A Level: Chemistry 9701/42 October/November 2020Zubair KhanNoch keine Bewertungen
- Two Tests For The Detection of Volatile Organic Acids and FormaldehydeDokument7 SeitenTwo Tests For The Detection of Volatile Organic Acids and Formaldehyde38221Noch keine Bewertungen
- Assignment2 Solution CIVE207 W24Dokument11 SeitenAssignment2 Solution CIVE207 W24tasnim.tanvir99Noch keine Bewertungen
- Subjects Carrying A Total of 600 Marks To Be SelectedDokument29 SeitenSubjects Carrying A Total of 600 Marks To Be SelectedMirza BaigNoch keine Bewertungen
- Peter G. NELSON: Modified Lewis TheoryDokument4 SeitenPeter G. NELSON: Modified Lewis Theorych_ymyaaNoch keine Bewertungen
- Chemistry For Engineers Set ADokument5 SeitenChemistry For Engineers Set AMark Jecel RapirNoch keine Bewertungen
- SQP1Dokument10 SeitenSQP1The. Daksh SharmaNoch keine Bewertungen
- Wolf - SR - Formation and Stability of Complex Metallic Phases Including QC Explored Through Combinatorial MethodsDokument11 SeitenWolf - SR - Formation and Stability of Complex Metallic Phases Including QC Explored Through Combinatorial MethodsAlisson WintherNoch keine Bewertungen
- R.T.-1 P-08-05-2011 Paper-1 12th (ABCD) (English) Code-B WADokument12 SeitenR.T.-1 P-08-05-2011 Paper-1 12th (ABCD) (English) Code-B WASushmit GuptaNoch keine Bewertungen
- Physics Quiz 11 20 Source1Dokument103 SeitenPhysics Quiz 11 20 Source1naborcarleugeneNoch keine Bewertungen
- Chapter 3Dokument6 SeitenChapter 3Katsuki HashimotoNoch keine Bewertungen
- Green ChemDokument80 SeitenGreen ChemMust LikeNoch keine Bewertungen
- Piping Colour Code Is 2379Dokument3 SeitenPiping Colour Code Is 2379Yashpal Singh Ghaman100% (2)
- Thesis Huifei JinDokument170 SeitenThesis Huifei Jintaufiqishak09Noch keine Bewertungen
- Dreyer Analysis 1988Dokument349 SeitenDreyer Analysis 1988Yutt WattNoch keine Bewertungen
- h3 BasicDokument11 Seitenh3 BasicBC AmrathaNoch keine Bewertungen
- 2 bài đọc hiểuDokument5 Seiten2 bài đọc hiểutrtlinh08Noch keine Bewertungen
- Plates & Shells Theories: Kirchhfoff Reissner/MindlinDokument32 SeitenPlates & Shells Theories: Kirchhfoff Reissner/MindlinNathaji ShelkeNoch keine Bewertungen