Beruflich Dokumente
Kultur Dokumente
230 S10 HW9
Hochgeladen von
pumjlffoOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
230 S10 HW9
Hochgeladen von
pumjlffoCopyright:
Verfügbare Formate
MSE 230
HW9 (due 03/25, 03/26)
Spring 2010
1. The important refractory ceramic MgO has the NaCl crystal structure. (a) Neglecting the small effect of CN on ionic radius, how much larger would the Mg2+ cation have to be in order for MgO to possibly have the CsCl crystal structure? (b) Based on equation 2.8 (including associated footnote), which factor, ion valence or ion separation (r = rcation + ranion), contributes more to the higher melting temperature of MgO compared to NaCl (Table 2.3)? Do not calculate absolute EA values, but show the relative effects using ratios. 2. One form of the important abrasive ceramic SiC has the ZnS crystal structure (see Fig. 12.4). (a) From an electronic structure standpoint, explain briefly why the coordination number in SiC is 4. (b) Calculate the lattice parameter of this structure based on the true density (pore-free) of 3.22 g/cm3. (c) Bulk SiC, like many bulk ceramics, often contains residual porosity (small voids) due to incomplete sintering. What volume fraction (i.e., 0 to 1) of porosity would be in a sintered SiC ceramic having a bulk density of 3.10 g/cm3? 3. Ceramics based on the Al2O3-SiO2 system (p. 442) are used for processing molten metals. The maximum use temperature is limited by the formation of any amount of liquid phase. (a) On a copy or tracing of the Al2O3-SiO2 phase diagram draw a heavy line indicating the maximum use temperature versus composition over the entire range. (b) Why is about 80 wt.% alumina the minimum alumina content in alumina-silica refractories for handling molten steel? 4. Five rectangular bend test bars 5 mm x 5 mm x 50 mm long are cut from a sintered silicon nitride and tested in 3-point bending according to Fig. 12.32 with L = 40 mm. The test bars fracture at the mid-point (L/2) under loads, Ff = 934, 1313, 1170, 850, and 1099 N, respectively. (a) Calculate the average flexural strength of this silicon nitride. (b) For the highest and lowest test result, estimate the size of the flaw that caused fracture, assuming surface cracks of length a, Y = 1.2 and the appropriate fracture toughness from Appendix B. (c) Explain briefly why the average strength would increase if the test specimen size were decreased. (d) Explain briefly why the average strength would decrease if the specimens were tested in uniaxial tension. 5. Thermal tempering is commonly used to strengthen glass components. (a) Describe the process of tempering glass and explain the origin(s) of the strengthening effect. (b) Compare and contrast the tempering of glass with the tempering of steel. 6. Callister 13.14: For many viscous materials, the viscosity may be defined in terms of the expression, = ,
d dt
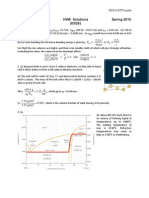
where and d/dt are, respectively, the tensile stress and (tensile) strain rate. A cylindrical specimen of borosilicate glass of diameter 4 mm and length 125 mm is subjected to a tensile force of 2 N along its axis. If its deformation (displacement) is to be less than 2.5 mm over a weeks time, using Fig. 13.7, determine the maximum temperature to which the specimen may be heated.
Das könnte Ihnen auch gefallen
- 230 S10 HW12 SolnsDokument2 Seiten230 S10 HW12 SolnspumjlffoNoch keine Bewertungen
- 230 S10 HW11Dokument1 Seite230 S10 HW11pumjlffoNoch keine Bewertungen
- 230 S10 HW10 SolnsDokument5 Seiten230 S10 HW10 SolnspumjlffoNoch keine Bewertungen
- 230 S10 HW11 SolnsDokument2 Seiten230 S10 HW11 SolnspumjlffoNoch keine Bewertungen
- 230 S10 HW10Dokument2 Seiten230 S10 HW10pumjlffoNoch keine Bewertungen
- MSE 230 HW12 (Due 04/22, 04/23) Spring 2010Dokument1 SeiteMSE 230 HW12 (Due 04/22, 04/23) Spring 2010pumjlffoNoch keine Bewertungen
- 230 S10 HW9 SolnsDokument2 Seiten230 S10 HW9 SolnspumjlffoNoch keine Bewertungen
- 230 S10 HW8Dokument1 Seite230 S10 HW8pumjlffoNoch keine Bewertungen
- 230 S10 HW7 SolnsDokument3 Seiten230 S10 HW7 SolnspumjlffoNoch keine Bewertungen
- 230 S10 HW6Dokument1 Seite230 S10 HW6pumjlffoNoch keine Bewertungen
- 230 S10 HW8 Solns0Dokument3 Seiten230 S10 HW8 Solns0pumjlffoNoch keine Bewertungen
- 230 S10 HW7Dokument2 Seiten230 S10 HW7pumjlffoNoch keine Bewertungen
- 230 S10 HW5 SolutionsDokument2 Seiten230 S10 HW5 SolutionspumjlffoNoch keine Bewertungen
- 230 S10 HW6 Solns0Dokument3 Seiten230 S10 HW6 Solns0pumjlffoNoch keine Bewertungen
- 230 S10 HW2Dokument1 Seite230 S10 HW2pumjlffoNoch keine Bewertungen
- 230 S10 HW5Dokument1 Seite230 S10 HW5pumjlffoNoch keine Bewertungen
- MSE 230 HW4 (Due 02/4, 02/5) Spring 2010Dokument1 SeiteMSE 230 HW4 (Due 02/4, 02/5) Spring 2010pumjlffoNoch keine Bewertungen
- 230 S10 HW3Dokument1 Seite230 S10 HW3pumjlffoNoch keine Bewertungen
- MSE 230 HW1 (Due 01/14, 01/15) Spring 2010Dokument1 SeiteMSE 230 HW1 (Due 01/14, 01/15) Spring 2010pumjlffoNoch keine Bewertungen
- 230 S10 HW4 SolutionsDokument2 Seiten230 S10 HW4 SolutionspumjlffoNoch keine Bewertungen
- 230 S10 HW3 Solutions0Dokument5 Seiten230 S10 HW3 Solutions0pumjlffoNoch keine Bewertungen
- 230 S10 HW2 SolnsDokument4 Seiten230 S10 HW2 SolnspumjlffoNoch keine Bewertungen
- 230 S10 HW1 Solns0Dokument3 Seiten230 S10 HW1 Solns0pumjlffoNoch keine Bewertungen
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (121)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- Sai Deepa Rock Drills: Unless Otherwise Specified ToleranceDokument1 SeiteSai Deepa Rock Drills: Unless Otherwise Specified ToleranceRavi BabaladiNoch keine Bewertungen
- Astn/Ason and Gmpls Overview and Comparison: By, Kishore Kasi Udayashankar Kaveriappa Muddiyada KDokument44 SeitenAstn/Ason and Gmpls Overview and Comparison: By, Kishore Kasi Udayashankar Kaveriappa Muddiyada Ksrotenstein3114Noch keine Bewertungen
- Riveted JointsDokument28 SeitenRiveted Jointsgnbabuiitg0% (1)
- Yz125 2005Dokument58 SeitenYz125 2005Ignacio Sanchez100% (1)
- Email ID: Contact No: +971562398104, +917358302902: Name: R.VishnushankarDokument6 SeitenEmail ID: Contact No: +971562398104, +917358302902: Name: R.VishnushankarJêmš NavikNoch keine Bewertungen
- Handbook+for+Participants+ +GCC+TeenDokument59 SeitenHandbook+for+Participants+ +GCC+Teenchloe.2021164Noch keine Bewertungen
- 2014 Abidetal. TheoreticalPerspectiveofCorporateGovernance BulletinofBusinessDokument11 Seiten2014 Abidetal. TheoreticalPerspectiveofCorporateGovernance BulletinofBusinessOne PlusNoch keine Bewertungen
- Bearing TypesDokument5 SeitenBearing TypesWayuNoch keine Bewertungen
- Graduate Macro Theory II: The Real Business Cycle Model: Eric Sims University of Notre Dame Spring 2017Dokument25 SeitenGraduate Macro Theory II: The Real Business Cycle Model: Eric Sims University of Notre Dame Spring 2017Joab Dan Valdivia CoriaNoch keine Bewertungen
- ARCASIA Students Design Competition TORDokument4 SeitenARCASIA Students Design Competition TORDeena McgeeNoch keine Bewertungen
- EE 8602 - Protection and Switchgear Unit I - MCQ BankDokument11 SeitenEE 8602 - Protection and Switchgear Unit I - MCQ Bankpoonam yadavNoch keine Bewertungen
- Zilog Z80-SIO Technical Manual TextDokument58 SeitenZilog Z80-SIO Technical Manual Textprada.rizzoplcNoch keine Bewertungen
- Yumemiru Danshi Wa Genjitsushugisha Volume 2Dokument213 SeitenYumemiru Danshi Wa Genjitsushugisha Volume 2carldamb138Noch keine Bewertungen
- EE360 - Magnetic CircuitsDokument48 SeitenEE360 - Magnetic Circuitsبدون اسمNoch keine Bewertungen
- Num Sheet 1Dokument1 SeiteNum Sheet 1Abinash MohantyNoch keine Bewertungen
- EF3e Intplus Filetest 10aDokument4 SeitenEF3e Intplus Filetest 10aLin Shufen100% (1)
- The Invisible SunDokument7 SeitenThe Invisible SunJay Alfred100% (1)
- Florida Motor Fuel Tax Relief Act of 2022Dokument9 SeitenFlorida Motor Fuel Tax Relief Act of 2022ABC Action NewsNoch keine Bewertungen
- Pressure-Dependent Leak Detection Model and Its Application To A District Water SystemDokument13 SeitenPressure-Dependent Leak Detection Model and Its Application To A District Water SystemManjul KothariNoch keine Bewertungen
- 3.15.E.V25 Pneumatic Control Valves DN125-150-EnDokument3 Seiten3.15.E.V25 Pneumatic Control Valves DN125-150-EnlesonspkNoch keine Bewertungen
- Tools of Persuasion StudentsDokument4 SeitenTools of Persuasion StudentsBelén Revilla GonzálesNoch keine Bewertungen
- Motive 27Tmx: Data SheetDokument2 SeitenMotive 27Tmx: Data SheetUlisesGómezNoch keine Bewertungen
- Excavation Trench Permit Ex 1 F0206Dokument5 SeitenExcavation Trench Permit Ex 1 F0206emeka2012Noch keine Bewertungen
- Gr. 10 Persuasive EssayDokument22 SeitenGr. 10 Persuasive EssayZephania JandayanNoch keine Bewertungen
- IPHPDokument4 SeitenIPHPAliah CasilangNoch keine Bewertungen
- Kimura K.K. (KKK) : Can This Customer Be Saved? - Group D13Dokument6 SeitenKimura K.K. (KKK) : Can This Customer Be Saved? - Group D13Mayuresh GaikarNoch keine Bewertungen
- Disney - QMDokument14 SeitenDisney - QMSyarifuddin Zulkifli0% (1)
- Lifecycle of A Butterfly Unit Lesson PlanDokument11 SeitenLifecycle of A Butterfly Unit Lesson Planapi-645067057Noch keine Bewertungen
- A Guide To Sample Size For Animal-Based Studies (VetBooks - Ir)Dokument292 SeitenA Guide To Sample Size For Animal-Based Studies (VetBooks - Ir)Jonathan MannNoch keine Bewertungen
- An Overview and Framework For PD Backtesting and BenchmarkingDokument16 SeitenAn Overview and Framework For PD Backtesting and BenchmarkingCISSE SerigneNoch keine Bewertungen