Beruflich Dokumente
Kultur Dokumente
Enzymes Formal Report
Hochgeladen von
Jenelle Jane QuilanetaOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Enzymes Formal Report
Hochgeladen von
Jenelle Jane QuilanetaCopyright:
Verfügbare Formate
Effects of Change in pH and Temperature on the Reaction Rates of Enzyme-Catalyzed Reaction Marcus Natividad, Barbara Ngo, Lexley Ong
and Jane Jenelle Quilaneta Group 8 2C Pharmacy Biochemistry Laboratory ABSTRACT
In this experiment, the glucose assay using a DNS colorimetric method measured and showed the maximum capacity of invertase activity in increasing concentration of the standard through a graphical representation, the best fit straight line. Factors that affect the invertase is also investigated such as pH and temperature .The results obtained were represented by bellshaped graphs that shows the optimum amount of pH and temperature.
INTRODUCTION This experiment aims to make the students able to determine the effects of change in pH and temperature on the reaction rates of an enzymecatalyzed reaction. Living organisms are composed of intricately systems of chemical reaction. Most of these chemical reactions are catalyzed by enzymes that allow these reactions to proceed at a rate sufficient to sustain life. Enzyme accelerates reactions without being changed themselves [1]. It is protein molecule that is a biological catalyst with three characteristics. First, which is its basic function is to increase the rate of a reaction. Second, most enzymes act specifically with only one reactant called a substrate to produce products. The third and most remarkable characteristic is that enzymes are regulated from a state of low activity to high activity and vice versa [2]. Sucrose, commonly known as table sugar, is a disaccharide composed of an alpha-D-glucose molecule and a beta-D-fructose molecule linked by an alpha-1,4-glycosidic bond. When this bond is cleaved in a hydrolysis reaction, an equimolar mixture of glucose and fructose is generated. This mixture of monosaccharide is called invert sugar, which is derived from the fact that sucrose rotates plane polarized light to the right i.e., dextrorotatory, +66.5, whereas the hydrolysis products rotates plane polarized light to the left i.e., levorotatory, -20 . Sucrose can be hydrolyzed in the presence of an enzyme called invertase or sucrose which shown in the equation: Sucrose + H2O ---> glucose + fructose The official name for invertase is betafructofuranosidase, which implies that the reaction catalyzed by this enzyme is the hydrolysis of the terminal non-reducing betafructofuranoside residues in betafructofuranosides[3].
Invertase is usually found on plants. It acts as a catalyst for the hydrolysis of sucrose. It is a significant enzyme because glucose is an important product of photosynthesis. Invertase is also used in the confectionery industry where fructose is preferred over sucrose because it is sweeter and does not crystallize easily[4]. EXPERIMENTAL A. Sample Used Sample Used: Glucose, Dinitrosalicyclic reagent B. Procedures 1. Sucrose Assay Using Dinitrosalicyclic Method First, We prepared series of test tubes.All these test tubes were covered with marbles to prevent evaporation of solvent.Then, we added 3 drops (approximately 0.05ml) concentrated HCl to each tube and mixed it. It was incubated at 90C water bath for 5 minutes. Then an addition of 0.15 mL of KOH was done to neutralize the solution. Next, is the addition of 1.5 mL of 0.1 M buffer solution, pH 5 and was mixed.Then , 3 mL of DNS reagent was added. The test tubes were immersed in a 95C water bath for 10 minutes to develop the characteristic red-brown color. After the solution has been cooled, the absorbance at 540nm was measured. Finally, the hydrolizedsucrose standard curve was constructed by plotting A540 against concentration (mg/mL). 2. Effect of pH on Invertase Activity First, we prepared 6 numbered test tubes and a blank test tube. Then, we added 1.4 mL appropriate 0.1 M buffer solution to each test tube. For the blank test tube we just added 3mL of distilled water, for test tube number 1 we added pH 1.00, for test tube number 2 we added pH 3.00, for test tube number 3 we added pH 5.00, for test tube number 4 we added pH 7.00, for test tube number 5 we added pH 9.00 and for test tube number 6 we added pH 11.00. After adding the desired amount of pH to each test tube, 0.10 mL enzyme stock solution was added to each test tube except for the blank test tube.
It was mixed thoroughly and was incubated in 60C water bath for 5 minutes. Next, is the addition 1.50 mL of sucrose solution and was incubated reaction mixture in 60C water bath for 5 minutes. Then, 3 mL of Dinitrosalicyclic reagent was added. The test tubes were immersed in 5C water bath for 10 minutes to develop the characteristic red-brown color. Then the absorbance at 540 nm was measured. Finally, the amount of sucrose hydrolyzed was determined using hydrolyzed-sucrose standard curve that was constructed in the Dinitrosalicyclic colorimetric method. 3. Effect of Temperature on Invertase Activity To test the effect of temperature on Invertase activity, our group placed 20, 30, 50, 60, 70 and 90C water baths and prepared 6 test tubes with each test tube containing 1.5 mL sucrose solution. The test tubes were incubated separately for 5 minutes in each water bath. In another test tube, 0.80 mL enzyme stock solution was mixed with 19.20 mL 0.1M buffer solution,pH 5. Then, we added 3 mL of diluted enzyme solution to all tubes. It was incubated for another 5 minutes. Remember not to remove the test tubes from their respective water baths. Then, 3 mL of DNS reagent was added. The test tubes were immersed in a 95C water bath for 10 minutes to develop characteristic re-brown color and then it was cooled down. Remember that all the test tubes are covered with marble to prevent evaporation of solvent. Meanwhile, another member prepared a bank solution by repeating all the steps but instead of adding enzyme stock solution, denatured enzyme is used. Then, the absorbance at 540nm is measured. The amount of sucrose hydrolyzed was determined by using sucrose standard curve constructed in the dinitrosalicylic colometric method. RESULTS AND DISCUSSION Glucose, and other reducing sugars,reacts with DNS by oxidation reaction producing a colored compound that shows a maximal molar extinction at 530nm.Glucose is reduced into gluconic acid,same as to dinitrosalicylic acid to 3-amino,5-nitro saliccycli acid,giving the characterisctic red-brown color. Oxidation is a reversible chemical reaction in which one of the reactions is oxidation and the reverse reaction is reduction. Since the absorbance at 540 nm is linearly dependent on the concentration or mass of glucose the reaction can be used for quantification of reducing sugar In table 1, We were able to measure the amount of light transmitted through a sample at a given wavelength. The absorbance of blank test tube was subtracted to the
absorbance of the numbered test tubes. This was made to measure only the absorbance of the invertase, without the additional reagents.
Test tube no Amount of Acid hydrolyzed glucose (mg/ml)
Absorbance
blank 1 2 3 4 5 6 Table 1.Glucose Method
0 0.5 0.83 1.33 1.67 2.17 2.50 Assay Using
2.35 2.10-2.35 =-0.25 2.96-2.35 =0.61 3.04-2.35 =0.69 3.07-2.35 =0.72 3.10-2.35 =0.78 3.11-2.35 =0.76 Dinitrosalicyclic
The graph below is the glucose calibration curve. It was shows the presence of glucose in the solution and the rate of formation of glucose. It is illustrated that the absorbance is directly proportioned to the amount of hydrolyzed glucose which means that the higher the absorbance the higher amount of acid hydrolyzed glucose is present.
1 0.8 Absorbance 0.6 0.4 0.2 0 0
Glucose Assay
Glucose mg/ ml Figure 2. Linear Graph of Glucose Assay Using Dinitrosalicylic Method The Invertase can be affected by the changes in pH. An Extremely high or low pH values generally result in complete loss of activity for most enzymes.
pH
Amount of Acid hydrolyzed sucrose (mg/ml) 0.5
Absorbance 30 0.1
=1.126 1.459-0.213 =1.246 50 0.12 1.52-0.213 =1.367 60 0.32 2.88-0.213 =2.667 70 0.18 1.799-0.213 =1.586 90 0.17 1.762-0.213 =1.549 Table 3. Effect of Temperature on Invertase activity Optimum Temperature was obtained at 60C . It infers that the activation energy is spontaneous that the transition state was inferred to the highest value of absorbance in the graph. The highest peak of the curve represents the highest concentration which was determined by the Absorbance. Temperature above 60C shows that the invertase reached denaturation. Effect of temperature 0.35 0.3 mg/ml/min 0.25 0.2
blank 3 4 5 7 8 11 Table
0.223 A
1.652-0.233 =1.419 A 0.34 1.652-0.233 =0.032 A 0.28 1.580-0.233 =1.347 A 0.36 1.660-2.33 =1.427 A 0.26 1.601-0.233 =1.368 0.02 1.459-0.233 =1.226 2. Effect of pH on Invertase Activity
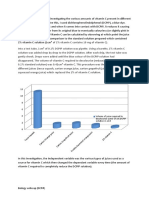
In figure 3, A bell shaped graph was formed. The most favorable pH value, which is the point where the enzyme is most active, is known as the optimum pH is at pH 6. It shows that at pH 7 and above it the concentration of invertase will slope down indicating denaturation of enzyme. It indicates that invertase reactivity is controlled and occurs to alimited extent. Effects of pH 1.4 1.2 mg/mL/min 1 0.8 0.6 0.4 0.2 0 0 5 pH 10 15
0.15 0.1
0.05 0 0 50 Temperature 100
Figure 4. Graph of Temperature on Invertase Activity REFERENCES [1]Crisostomo A. et.al.(2010).Laboratory Manual in General Biochemistry.Quezon City: C&E Publishing Inc.do.edu/hndbksupport/ochemlabtech.html 2003 [2]http://www.elmhurst.edu/~chm/vchembook/5 70enzymes.html [3]http://www.eng.umd.edu/~nsw/ench485/lab1 4.htm [4]Campbell N.A.;Reece J.B..(2002).Biology,6thedition.San Francisco,USA: Benjamin Cummings.
Figure 3. Graph of the effect of pH on Invertase Activity The temperature affects the invertase activity. Too cool will not allow the enzyme to react, Too hot will denature the protein.
Temperature
20
Amount of Acid hydrolyzed sucrose (mg/ml) 0.05
Absorbance
1.339-0.213
Das könnte Ihnen auch gefallen
- UST Faculty of Pharmacy Alkaloids ClassificationDokument8 SeitenUST Faculty of Pharmacy Alkaloids ClassificationJenelle Jane Quilaneta100% (1)
- Examples of AlkaloidsDokument7 SeitenExamples of AlkaloidsJenelle Jane QuilanetaNoch keine Bewertungen
- Wrist: by Hossein Khorrami, PH.D, DOMPDokument27 SeitenWrist: by Hossein Khorrami, PH.D, DOMPkhorrami4Noch keine Bewertungen
- Vitamin CDokument4 SeitenVitamin CAkshith Swamy MiriyalaNoch keine Bewertungen
- Lab Report AnatomyDokument7 SeitenLab Report Anatomyapi-339983508Noch keine Bewertungen
- Enzymes: Biological CatalystsDokument9 SeitenEnzymes: Biological CatalystsChamzelleNoch keine Bewertungen
- Ex 4 Effect of PH On Enzyme ActivityDokument2 SeitenEx 4 Effect of PH On Enzyme ActivityRochel CaduyacNoch keine Bewertungen
- Homeostasis Lab Effects Exercise Heart RateDokument4 SeitenHomeostasis Lab Effects Exercise Heart Ratethe cornchip executionerNoch keine Bewertungen
- Alkene Reactions with Bromine and Sulfuric AcidDokument11 SeitenAlkene Reactions with Bromine and Sulfuric AcidEdwin Lan100% (1)
- Effects of Temperature and PH On The Enzymatic Activity of Salivary Amylase - JAENDokument12 SeitenEffects of Temperature and PH On The Enzymatic Activity of Salivary Amylase - JAENCara JaenNoch keine Bewertungen
- Homeostasis Activity - MARY SHESHIRADokument3 SeitenHomeostasis Activity - MARY SHESHIRASheshira Reddy YeruvaNoch keine Bewertungen
- CHEM 22161 Lab 1 :synthesis of Medicinal Agent: Synthesis of AspirinDokument8 SeitenCHEM 22161 Lab 1 :synthesis of Medicinal Agent: Synthesis of AspirinKasun WekasingheNoch keine Bewertungen
- Amylase Experiment Lab ReportDokument7 SeitenAmylase Experiment Lab ReportCHLOE IANNAH CALVADORESNoch keine Bewertungen
- 1.2 IntermolecularDokument6 Seiten1.2 IntermolecularJade RanteNoch keine Bewertungen
- Amylase Assay 2Dokument9 SeitenAmylase Assay 2Rahman ImudaNoch keine Bewertungen
- Copper Determination in Water by Standard Addition PotentiometryDokument4 SeitenCopper Determination in Water by Standard Addition PotentiometryAura Ballesteros MontealegreNoch keine Bewertungen
- Quantitative Analysis Nelson's AssayDokument4 SeitenQuantitative Analysis Nelson's AssayJenelle Jane Quilaneta25% (4)
- AP Lab #2: Enzyme Catalysis LabDokument4 SeitenAP Lab #2: Enzyme Catalysis Labpointweb50% (2)
- How Does Exercise Affect Heart Rate Alvin PDFDokument13 SeitenHow Does Exercise Affect Heart Rate Alvin PDFapi-134712575Noch keine Bewertungen
- Vitamin C in Fruit JuicesDokument5 SeitenVitamin C in Fruit JuicesANMOL journeyNoch keine Bewertungen
- Chemistry Project 3.0Dokument15 SeitenChemistry Project 3.0Lubna Khalid67% (3)
- DCPIP Write UpDokument2 SeitenDCPIP Write UpHashim Chishty0% (1)
- Bio ReportDokument8 SeitenBio ReportTharshini_Indr_6713Noch keine Bewertungen
- Amylase Activity and Starch DigestionDokument10 SeitenAmylase Activity and Starch DigestionJulioNoch keine Bewertungen
- HomeostasisDokument5 SeitenHomeostasisMaria Jayiera Alkiela Pe�alesNoch keine Bewertungen
- AS Biology Unit 3 Practical ExamDokument30 SeitenAS Biology Unit 3 Practical ExamShamaNoch keine Bewertungen
- CHEMISTRY ProjectDokument17 SeitenCHEMISTRY ProjectMerin MariamNoch keine Bewertungen
- Chapter 2.6 Aldehyde & KetoneDokument40 SeitenChapter 2.6 Aldehyde & Ketone0JTINGNoch keine Bewertungen
- Lysosomes PresentationDokument53 SeitenLysosomes PresentationCaroline Stephenson50% (2)
- Poster About CarbohydratesDokument1 SeitePoster About CarbohydratesAlisya AzamiNoch keine Bewertungen
- Bio 100 Osmosis Lab ReportDokument4 SeitenBio 100 Osmosis Lab Reportapi-249188694Noch keine Bewertungen
- Carbohydrates Lipids Proteins Nucleic Acids Water - QuestionsDokument43 SeitenCarbohydrates Lipids Proteins Nucleic Acids Water - QuestionslundaNoch keine Bewertungen
- Exp 1Dokument9 SeitenExp 1Amirul Ramlan100% (1)
- LAB GravimetricAnalysisSulfateMixtureDokument3 SeitenLAB GravimetricAnalysisSulfateMixtureDNoch keine Bewertungen
- Investigate The Effect of PH On The Rate of Amylase EnzymeDokument2 SeitenInvestigate The Effect of PH On The Rate of Amylase EnzymeSmith PennanNoch keine Bewertungen
- Segs-Chemistry of Life: Problem SetDokument4 SeitenSegs-Chemistry of Life: Problem SetMelindaSuitosColobong-GarpidaNoch keine Bewertungen
- The Oxidation of Ascorbic Acid and Its Reduction in Vitro and in VivoDokument43 SeitenThe Oxidation of Ascorbic Acid and Its Reduction in Vitro and in VivoLuis J. RomeroNoch keine Bewertungen
- Le Chatelier's PrincipleSTDokument4 SeitenLe Chatelier's PrincipleSTDerek JohnsonNoch keine Bewertungen
- Effect of Temperature On Enzyme Kinetics StudyDokument7 SeitenEffect of Temperature On Enzyme Kinetics StudyYvonne MunNoch keine Bewertungen
- Factors That Influence Enzyme Lab ActivityDokument5 SeitenFactors That Influence Enzyme Lab ActivityDaniel BelnapNoch keine Bewertungen
- Determination of Ka of Weak AcidsDokument4 SeitenDetermination of Ka of Weak AcidsJohanson Bombaes100% (6)
- Red Blood Cell CountDokument4 SeitenRed Blood Cell CountMohamed MokhtarNoch keine Bewertungen
- CellsDokument20 SeitenCellsppttppNoch keine Bewertungen
- Carbohydrates: Color Reactions and TestsDokument19 SeitenCarbohydrates: Color Reactions and TestsAjith KumarNoch keine Bewertungen
- PBLDokument5 SeitenPBLlourdes kusumadiNoch keine Bewertungen
- 2 CLab ManualDokument129 Seiten2 CLab ManualMomerNoch keine Bewertungen
- Cell and Tissue CharacteristicsDokument12 SeitenCell and Tissue Characteristicsgreenflames09100% (1)
- COURSE WORK MOLECULAR BIOLOGY & GeneticsDokument3 SeitenCOURSE WORK MOLECULAR BIOLOGY & Geneticsusaeed00000Noch keine Bewertungen
- Manual Biology StudentDokument22 SeitenManual Biology StudentMalvinder Singh DhillonNoch keine Bewertungen
- Homeostasis: History Etymology Controls of VariablesDokument19 SeitenHomeostasis: History Etymology Controls of VariablesvirginiaNoch keine Bewertungen
- ChemistryDokument2 SeitenChemistryLulu Tojeen0% (1)
- Chap69 PDFDokument3 SeitenChap69 PDFIkrar SyahmarNoch keine Bewertungen
- Practical 2Dokument8 SeitenPractical 2Ibrahim Muhamad0% (1)
- Simultaneous Determination of Salicylic Acid and Acetylsalicylic AciDokument5 SeitenSimultaneous Determination of Salicylic Acid and Acetylsalicylic Aciiabureid7460Noch keine Bewertungen
- Diffusion and Osmosis in The Human BodyDokument13 SeitenDiffusion and Osmosis in The Human BodyCody EllisNoch keine Bewertungen
- Preparation of Tetraamminecopper II Sulphate.Dokument10 SeitenPreparation of Tetraamminecopper II Sulphate.DaizLee Ahmad25% (4)
- Lab Report For MonossacharideDokument15 SeitenLab Report For MonossacharideSay Cheez100% (1)
- Acid Base Tit RationsDokument11 SeitenAcid Base Tit RationsSandjaya EdmondNoch keine Bewertungen
- The Extraction of Invertase From Yeast and Its Effects On PH and TemperatureDokument5 SeitenThe Extraction of Invertase From Yeast and Its Effects On PH and TemperatureDeanne Louise Dela Cruz100% (2)
- Effect of Temp On EnzymesDokument3 SeitenEffect of Temp On EnzymesJanick MallareNoch keine Bewertungen
- Formal Report2Dokument5 SeitenFormal Report2Krisha VittoNoch keine Bewertungen
- TN Pharmacology in TablesDokument54 SeitenTN Pharmacology in Tablessakagucci6333Noch keine Bewertungen
- (2013) CPG - Psoriasis-2Dokument78 Seiten(2013) CPG - Psoriasis-2Jenelle Jane QuilanetaNoch keine Bewertungen
- Sparkle: ViolinDokument8 SeitenSparkle: ViolinJenelle Jane QuilanetaNoch keine Bewertungen
- Phytochemical Screening TestsDokument1 SeitePhytochemical Screening TestsJenelle Jane QuilanetaNoch keine Bewertungen
- Cerebral Infarct RadioDokument35 SeitenCerebral Infarct RadioJenelle Jane QuilanetaNoch keine Bewertungen
- Isolate and differentiate enteric bacteriaDokument24 SeitenIsolate and differentiate enteric bacteriaJenelle Jane QuilanetaNoch keine Bewertungen
- Pharmacology Anticancer DrugsDokument3 SeitenPharmacology Anticancer DrugsJenelle Jane QuilanetaNoch keine Bewertungen