Beruflich Dokumente
Kultur Dokumente
The Parathyroid Glands
Hochgeladen von
Ruth AlooOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
The Parathyroid Glands
Hochgeladen von
Ruth AlooCopyright:
Verfügbare Formate
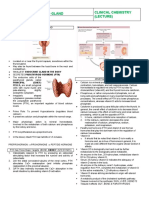
THE PARATHYROID GLANDS
Anatomy
Humans usually have four parathyroid glands: two embedded in the superior poles of the thyroid and two in its inferior poles (Figure 219). However, the locations of the individual parathyroids and their number can vary considerably. Parathyroid tissue is sometimes found in the mediastinum.
Figure 219.
The human parathyroid glands, viewed from behind.
Each parathyroid gland is a richly vascularized disk, about 3 x 6 x 2 mm, containing two distinct types of cells. The abundant chief cells, which contain a prominent Golgi apparatus plus endoplasmic reticulum and secretory granules (Figure 2110), synthesize and secrete parathyroid hormone (PTH). The less
abundant and larger oxyphil cells contain oxyphil granules and large numbers of mitochondria in their cytoplasm. In humans, few are seen before puberty, and thereafter they increase in number with age. Their function is unknown, although some investigators have argued that they are degenerated chief cells.
Actions
PTH acts directly on bone to increase bone resorption and mobilize Ca2+. In addition to increasing the plasma Ca2+ and depressing the plasma phosphate, PTH increases phosphate excretion in the urine. This phosphaturic action is due to a decrease in reabsorption of phosphate in the proximal tubules. PTH also increases reabsorption of Ca2+ in the distal tubules, although Ca2+ excretion is often increased in hyperparathyroidism because the increase in the amount filtered overwhelms the effect on reabsorption. PTH also increases the formation of 1,25-dihydroxycholecalciferol, and this increases Ca2+ absorption from the intestine. On a longer timescale, PTH stimulates both osteoblasts and osteoclasts. The net effect varies, but with mildly elevated plasma PTH levels it is usually anabolic.
Mechanism of Action
Figure 2112.
Signal transduction pathways activated by PTH or PTHrP binding to the hPTH/hPTHrP receptor. Intracellular cAMP is increased via Gs and adenylyl cyclase (AC). Diacylglycerol and IP3 (1,4,5-InsP3) are increased via Gq and phospholipase C (PLC). (Modified and reproduced, with permission, from McPhee SJ, Lingappa VR, Ganong WF [editors]: Pathophysiology of Disease, 4th ed. McGraw-Hill, 2003.)
In the disease called pseudohypoparathyroidism, the signs and symptoms of hypoparathyroidism develop but the circulating level of PTH is normal or elevated. Since the tissues fail to respond to the hormone, this is a receptor disease. There are two forms. In the more common form, a congenital 50% reduction of the activity of Gs occurs, and PTH fails to produce a normal increase in cAMP concentration. In a different, less common form, the cAMP response is normal but the phosphaturic action of the hormone is defective.
Regulation of Secretion
Circulating ionized calcium acts directly on the parathyroid glands in a negative feedback fashion to regulate the secretion of PTH (Figure 2113). The key to this regulation is a cell membrane Ca2+ receptor. This serpentine receptor is coupled via a G protein to phosphoinositide turnover and is found in many tissues. In the parathyroid, its activation inhibits PTH secretion. In this way, when the plasma Ca 2+ level is high, PTH secretion is inhibited and the Ca2+ is deposited in the bones. When it is low, secretion is increased and Ca2+ is mobilized from the bones.
Figure 2113.
Relation between plasma Ca2+ concentration and PTH response in humans. The set point is the plasma Ca2+ at which half the maximal response occurred. (Modified and reproduced, with permission, from Brown E: Extracellular Ca2+ sensing, regulation of parathyroid cell functions, and role of Ca2+ and other ions as extracellular (first) messengers. Physiol Rev 1991;71:371.)
1,25-Dihydroxycholecalciferol acts directly on the parathyroid glands to decrease preproPTH mRNA. Increased plasma phosphate stimulates PTH secretion by lowering plasma Ca2+ and inhibiting the formation of 1,25-dihydroxycholecalciferol. Magnesium is required to maintain normal parathyroid secretory responses. Impaired PTH release along with diminished target organ responses to PTH account for the hypocalcemia that occasionally occurs in magnesium deficiency.
Effects of Parathyroidectomy
PTH is essential for life. After parathyroidectomy, there is a steady decline in the plasma Ca 2+ level. Signs of neuromuscular hyperexcitability appear, followed by full-blown hypocalcemic tetany (see above). Plasma phosphate levels usually rise as the plasma calcium level falls after parathyroidectomy, but the rise does not always occur. In humans, tetany is most often due to inadvertent parathyroidectomy during thyroid surgery. Symptoms usually develop 23 days postoperatively but may not appear for several weeks or more. In rats on a lowcalcium diet, tetany develops much more rapidly, and death occurs 610 hours after parathyroidectomy. Injections of PTH correct the chemical abnormalities, and the symptoms disappear. Injections of Ca 2+ salts give temporary relief. The signs of tetany in humans include Chvostek's sign, a quick contraction of the ipsilateral facial
muscles elicited by tapping over the facial nerve at the angle of the jaw; and Trousseau's sign, a spasm of the muscles of the upper extremity that causes flexion of the wrist and thumb with extension of the fingers (Figure 2114). In individuals with mild tetany in whom spasm is not evident, Trousseau's sign can sometimes be produced by occluding the circulation for a few minutes with a blood pressure cuff.
Figure 2114.
Position of the hand in hypocalcemic tetany (Trousseau's sign).
Parathyroid Hormone Excess
Hyperparathyroidism due to injections of parathyroid extract in animals or hypersecretion of a functioning parathyroid tumor in humans is characterized by hypercalcemia and hypophosphatemia. Humans with PTH-secreting adenomas are usually asymptomatic, with the condition detected when plasma Ca2+ is measured in conjunction with a routine physical examination. However, there may be minor changes in personality, and calcium-containing kidney stones occasionally form.
Secondary Hyperparathyroidism
In conditions such as chronic renal disease and rickets, in which the plasma Ca2+ level is chronically low, stimulation of the parathyroid glands causes compensatory parathyroid hypertrophy and secondary hyperparathyroidism. The plasma Ca2+ level is low in chronic renal disease primarily because the diseased kidneys lose the ability to form 1,25-dihydroxycholecalciferol.
Familial Hypercalcemia & Hypocalcemia
Mutations in the gene for the Ca2+ receptor cause predictable long-term changes in plasma Ca2+. Individuals heterozygous for inactivating mutations have familial benign hypocalciuric hypercalcemia, a condition in which there is a chronic moderate elevation in plasma Ca2+ because the feedback inhibition of PTH secretion by Ca2+ is reduced. Plasma PTH levels are normal or even elevated. However, children who are homozygous for inactivating mutations develop neonatal severe primary hyperparathyroidism. Individuals with gain-of-function mutations of the gene for the Ca2+ receptor develop familial hypercalciuric hypocalcemia due to increased sensitivity of the parathyroid glands to plasma Ca2+.
PTHrP
A protein with PTH activity, parathyroid hormone-related protein (PTHrP), is produced by many different tissues in the body. It has 140 amino acid residues, compared with 84 in PTH, and is encoded by a gene on human chromosome 12, whereas PTH is encoded by a gene on chromosome 11. However, PTHrP and PTH have marked homology at their amino terminal ends, with 8 of the first 13 amino acid residues in the same positions, and they both bind to the hPTH/ PTHrP receptor. Yet their physiologic effects are very different. How is this possible when they bind to the same receptor? For one thing, PTHrP is primarily a tissue hormone or factor, acting where it is produced. It may be that circulating PTH cannot reach these sites. Another possibility is action of one or the other hormone on other, more selective receptors. In any case, PTHrP has a marked effect on the growth and development of cartilage in utero. Mice in which both alleles of the PTHrP gene are knocked out (ie, those homozygous for the defect) have severe skeletal deformities and die soon after birth. In utero, when PTHrP is released, cartilage is stimulated by a protein called Indian hedgehog. The PTHrP-stimulated cartilage cells proliferate and their terminal differentiation is inhibited. PTHrP is also expressed in the brain, where evidence indicates that it inhibits excitotoxic damage to developing neurons. In addition, there is evidence that it is involved in Ca2+ transport in the placenta. Although homozygous PTHrP knockouts are lethal, it is possible to genetically engineer mice that survive because they have a local increase in PTHrP in their cartilage. In these animals, breasts fail to develop, a finding that is consistent with the observation that in normal lactating mice there is abundant PTHrP in the milk. However, the plasma level does not increase during lactation, and the function of PTHrP in milk is unknown. PTHrP is also found in keratinocytes in the skin, in smooth muscle, and in the teeth, where it is present in the enamel epithelium that caps each tooth. In the absence of PTHrP, teeth cannot erupt.
Hypercalcemia of Malignancy
Hypercalcemia is a common metabolic complication of cancer. About 20% of hypercalcemic patients have bone metastases that produce the hypercalcemia by eroding bone (local osteolytic hypercalcemia). Evidence suggests that this erosion is produced by prostaglandins such as PGE from the tumor. The hypercalcemia in the remaining 80% of the patients is due to elevated circulating levels of PTHrP
(humoral hypercalcemia of malignancy). The tumors responsible for the hypersecretion include cancers of the breast, kidney, ovary, and skin.
Das könnte Ihnen auch gefallen
- Physiology, Parathyroid Hormone (PTH) : Statpearls (Internet)Dokument10 SeitenPhysiology, Parathyroid Hormone (PTH) : Statpearls (Internet)chafeb febiNoch keine Bewertungen
- Pathophysiology Primary HyperparathyroidismDokument3 SeitenPathophysiology Primary HyperparathyroidismIronic SunNoch keine Bewertungen
- Para ThyroidDokument41 SeitenPara ThyroidAdham YounesNoch keine Bewertungen
- Hormones of Parathyroid and Regulation of Blood Calcium LevelDokument9 SeitenHormones of Parathyroid and Regulation of Blood Calcium Levelriskyy1Noch keine Bewertungen
- Parathyroid Glands: Serum PTH Levels Are Inappropriately Elevated For The LevelDokument4 SeitenParathyroid Glands: Serum PTH Levels Are Inappropriately Elevated For The LevelNada MuchNoch keine Bewertungen
- Tyroid 10Dokument6 SeitenTyroid 10Anonymous UTUWFODCEYNoch keine Bewertungen
- Tyroid 10Dokument4 SeitenTyroid 10Anonymous UTUWFODCEYNoch keine Bewertungen
- New Microsoft Office Word DocumentDokument11 SeitenNew Microsoft Office Word Documentrain_puvNoch keine Bewertungen
- Hypoparathyroidism / Hypocalcemia: Abrar Alharbi, F1Dokument32 SeitenHypoparathyroidism / Hypocalcemia: Abrar Alharbi, F1abrarNoch keine Bewertungen
- Hipercalcemia - F B MaterialDokument32 SeitenHipercalcemia - F B MaterialDenisa Carmen ColiofNoch keine Bewertungen
- 1-7 BaileysDokument7 Seiten1-7 BaileysAura NirwanaNoch keine Bewertungen
- Hiperparatiroid: Dr. Dr. Shahrul Rahman, SP - PD, FINASIMDokument50 SeitenHiperparatiroid: Dr. Dr. Shahrul Rahman, SP - PD, FINASIMBonitavanyNoch keine Bewertungen
- Hiper Hipoparatir NejmDokument13 SeitenHiper Hipoparatir NejmPriscila Tobar AlcántarNoch keine Bewertungen
- Endocrine System DR Yogesh Swami 3Dokument11 SeitenEndocrine System DR Yogesh Swami 3chiragm1408Noch keine Bewertungen
- The Calcium Regulating HormonesDokument57 SeitenThe Calcium Regulating Hormonesdrsanju17Noch keine Bewertungen
- Primary HyperparathyroidismDokument16 SeitenPrimary HyperparathyroidismHamza AhmedNoch keine Bewertungen
- PTH Regulation and Hyperparathyroidism GuideDokument39 SeitenPTH Regulation and Hyperparathyroidism GuideAris josuaNoch keine Bewertungen
- Characteristics and Treatment of Hypertension in PheochromocytomaDokument17 SeitenCharacteristics and Treatment of Hypertension in PheochromocytomaJunior TorresNoch keine Bewertungen
- Parathyroid Hormone MetabolismDokument7 SeitenParathyroid Hormone MetabolismAssault AmphibiansNoch keine Bewertungen
- Phosphorus Restriction Prevents Parathyroid Gland Growth: High Phosphorus Directly Stimulates PTH Secretion in VitroDokument7 SeitenPhosphorus Restriction Prevents Parathyroid Gland Growth: High Phosphorus Directly Stimulates PTH Secretion in VitroDaniel Lee Eisenberg JacobsNoch keine Bewertungen
- Central Hypothyroidism: Pathogenic, Diagnostic, and Therapeutic ChallengesDokument11 SeitenCentral Hypothyroidism: Pathogenic, Diagnostic, and Therapeutic ChallengesCroitort53Noch keine Bewertungen
- Parathyroid Hormone: DR Pramod Kumar Asstt. Professor Department of Veterinary Physiology Bihar Veterinary College, PatnaDokument19 SeitenParathyroid Hormone: DR Pramod Kumar Asstt. Professor Department of Veterinary Physiology Bihar Veterinary College, PatnasanathNoch keine Bewertungen
- Parathyroid DisordersDokument37 SeitenParathyroid DisordersMannat ZaidiNoch keine Bewertungen
- Somaia Noman Rema Alqadhi Labiba Mohammed Lamia AlkamaliDokument26 SeitenSomaia Noman Rema Alqadhi Labiba Mohammed Lamia AlkamaliAhmed AL-shaabiNoch keine Bewertungen
- Secondary hyperparathyroidism and renal failure causes of hypercalcemiaDokument4 SeitenSecondary hyperparathyroidism and renal failure causes of hypercalcemiajamil aoudeNoch keine Bewertungen
- Lecture Notes in Medical Technology - Lecture #6 - The PARATHYROID GLANDDokument15 SeitenLecture Notes in Medical Technology - Lecture #6 - The PARATHYROID GLANDKat JornadalNoch keine Bewertungen
- Secondary Hyperparathyroidism - Pathogenesis, Disease Progression, and Therapeutic OptionsDokument9 SeitenSecondary Hyperparathyroidism - Pathogenesis, Disease Progression, and Therapeutic OptionsJuanita GonzálezNoch keine Bewertungen
- Pediatric Endocrinology Part 2: Pediatrics 2Dokument8 SeitenPediatric Endocrinology Part 2: Pediatrics 2sarguss14Noch keine Bewertungen
- Rare pituitary adenomas and gonadotrophin deficiencyDokument6 SeitenRare pituitary adenomas and gonadotrophin deficiencyDirgantari PademmeNoch keine Bewertungen
- Pituitary GlandDokument31 SeitenPituitary GlandNeha BhartiNoch keine Bewertungen
- Paratiroid Dan KalsiumDokument80 SeitenParatiroid Dan KalsiumFebrina EvaNoch keine Bewertungen
- Disorders of The Parathyroid GlandsDokument30 SeitenDisorders of The Parathyroid Glandsikram ullah khan100% (1)
- Complicated MalariaDokument56 SeitenComplicated MalariaAmina MgunyaNoch keine Bewertungen
- Week 5: Endocrinology: I. Pituitary ControlDokument26 SeitenWeek 5: Endocrinology: I. Pituitary ControlBenjamin NgNoch keine Bewertungen
- Parathyroid Gland LessonDokument3 SeitenParathyroid Gland LessonCherry Ann ColechaNoch keine Bewertungen
- Eje 257Dokument15 SeitenEje 257Sam SoeteNoch keine Bewertungen
- Precocious PubertyDokument30 SeitenPrecocious PubertyAjoritsedere Eric AkonuNoch keine Bewertungen
- Pituitary DisorderDokument48 SeitenPituitary DisorderAyumi AgungNoch keine Bewertungen
- Guidelines of AsDokument9 SeitenGuidelines of AsLIZA2627428BNoch keine Bewertungen
- ProlactinomaDokument8 SeitenProlactinomaGaby Vega SalazarNoch keine Bewertungen
- Precocious PubertyDokument30 SeitenPrecocious PubertyNeha SharmaNoch keine Bewertungen
- Diagnosis and Management of HyperparathyroidismDokument20 SeitenDiagnosis and Management of Hyperparathyroidismpaingmyint100% (1)
- Excess Parathyroid Hormone Adversely Affects Lipid Metabolism in Chronic Renal FailureDokument5 SeitenExcess Parathyroid Hormone Adversely Affects Lipid Metabolism in Chronic Renal FailureJuni ClaudiaNoch keine Bewertungen
- PHP2018Dokument25 SeitenPHP2018radu nicolaeNoch keine Bewertungen
- Endocrine SystemDokument30 SeitenEndocrine SystemYary MayorNoch keine Bewertungen
- Endocrine Exam 1 ReviewDokument11 SeitenEndocrine Exam 1 ReviewJohn SmithNoch keine Bewertungen
- 21 CP Endocrine 2Dokument73 Seiten21 CP Endocrine 2Aman Singh RaoNoch keine Bewertungen
- Parathyroid Hormone: Shahab Ullah Khan Ayub Medical CollegeDokument28 SeitenParathyroid Hormone: Shahab Ullah Khan Ayub Medical CollegeDr KhanNoch keine Bewertungen
- Parathyroid and Adrenal Gland DisordersDokument29 SeitenParathyroid and Adrenal Gland DisordersifamhmudahNoch keine Bewertungen
- UW Notes - 8 - Endocrine ArrangeddDokument40 SeitenUW Notes - 8 - Endocrine Arrangeddmind blocNoch keine Bewertungen
- CJN 10390917 FullDokument10 SeitenCJN 10390917 FullAndrea ArbizuNoch keine Bewertungen
- New Directions in The Treatment of HypoparathyroidismDokument6 SeitenNew Directions in The Treatment of Hypoparathyroidismmiguel saba sabaNoch keine Bewertungen
- Elecsys PTH (1-84) : Cobas e 801 English System InformationDokument5 SeitenElecsys PTH (1-84) : Cobas e 801 English System Informationsyafiq_82Noch keine Bewertungen
- Jurnal 6Dokument21 SeitenJurnal 6onisNoch keine Bewertungen
- PITUITARYDokument37 SeitenPITUITARYaparna shamaNoch keine Bewertungen
- The Parathyroid GlandDokument66 SeitenThe Parathyroid GlandMohammed GamalNoch keine Bewertungen
- Pseudohypoparathyroidism-Literature Update 2018Dokument8 SeitenPseudohypoparathyroidism-Literature Update 2018radu nicolaeNoch keine Bewertungen
- Parathyroid Gland Diseases: Classification of Diseases of PTGDokument11 SeitenParathyroid Gland Diseases: Classification of Diseases of PTGgashbin latifNoch keine Bewertungen
- Management of Normocalcemic Primary HyperparathyroidismDokument19 SeitenManagement of Normocalcemic Primary HyperparathyroidismErika AvilaNoch keine Bewertungen
- Fast Facts: Déficit en pyruvate kinase: Sensibilisation à cette maladie génétique rareVon EverandFast Facts: Déficit en pyruvate kinase: Sensibilisation à cette maladie génétique rareBewertung: 4 von 5 Sternen4/5 (1)
- Viral PathogenesisDokument11 SeitenViral PathogenesisRuth AlooNoch keine Bewertungen
- Laws Affecting Pharmacy Practice in KenyaDokument92 SeitenLaws Affecting Pharmacy Practice in KenyaRuth AlooNoch keine Bewertungen
- Rheology & Flow of FluidsDokument1 SeiteRheology & Flow of FluidsRuth AlooNoch keine Bewertungen
- Sample Format For Reference WritingDokument1 SeiteSample Format For Reference WritingRuth AlooNoch keine Bewertungen
- Membrane and Excitable Tissue Physiology: The Nervous System (NS)Dokument3 SeitenMembrane and Excitable Tissue Physiology: The Nervous System (NS)Ruth AlooNoch keine Bewertungen
- Sterilization and DisinfectionDokument17 SeitenSterilization and DisinfectionRuth AlooNoch keine Bewertungen
- Powder TechnologyDokument30 SeitenPowder TechnologyRuth AlooNoch keine Bewertungen
- Drug Isolation ProcessesDokument5 SeitenDrug Isolation ProcessesRuth AlooNoch keine Bewertungen
- Synthesis of Cobaltoxime DerivativesDokument5 SeitenSynthesis of Cobaltoxime DerivativesRuth AlooNoch keine Bewertungen
- ConstitutionDokument4 SeitenConstitutionRuth AlooNoch keine Bewertungen
- Drug Isolation ProcessesDokument5 SeitenDrug Isolation ProcessesRuth AlooNoch keine Bewertungen
- Word 2000Dokument49 SeitenWord 2000Ruth AlooNoch keine Bewertungen
- ConstitutionDokument4 SeitenConstitutionRuth AlooNoch keine Bewertungen
- Insurance LiabilitiesDokument2 SeitenInsurance LiabilitiesRuth AlooNoch keine Bewertungen
- Biosynthesis AssignmentDokument7 SeitenBiosynthesis AssignmentRuth AlooNoch keine Bewertungen
- Chap 9 - Statins - Not Just AboutDokument1 SeiteChap 9 - Statins - Not Just AboutRuth AlooNoch keine Bewertungen
- c191 USP36Dokument3 Seitenc191 USP36Ruth AlooNoch keine Bewertungen
- DK2015 CH01Dokument15 SeitenDK2015 CH01kjinguy642Noch keine Bewertungen
- Abdomen Test QuestionsDokument20 SeitenAbdomen Test QuestionsRuth AlooNoch keine Bewertungen
- AssignmentDokument6 SeitenAssignmentRuth AlooNoch keine Bewertungen
- Anatomical Characteristics of The Vestibular SystemDokument7 SeitenAnatomical Characteristics of The Vestibular SystemRuth AlooNoch keine Bewertungen
- Cholestrol AssignmentDokument2 SeitenCholestrol AssignmentRuth AlooNoch keine Bewertungen
- Abdomen Test QuestionsDokument20 SeitenAbdomen Test QuestionsRuth AlooNoch keine Bewertungen
- Female Sexual ActDokument3 SeitenFemale Sexual ActRuth Aloo100% (1)
- Chap 2 - Aspirin - An Old Actor With A New RoleDokument1 SeiteChap 2 - Aspirin - An Old Actor With A New RoleRuth AlooNoch keine Bewertungen
- Chap 1 - Cutting Gordian KnotsDokument1 SeiteChap 1 - Cutting Gordian KnotsRuth AlooNoch keine Bewertungen
- Abdomen Test QuestionsDokument20 SeitenAbdomen Test QuestionsRuth AlooNoch keine Bewertungen
- Methemoglobinemia: Maryland Poison CenterDokument4 SeitenMethemoglobinemia: Maryland Poison CenterRuth AlooNoch keine Bewertungen
- Com 110 AssignmentDokument4 SeitenCom 110 AssignmentRuth AlooNoch keine Bewertungen
- Worksheet Modul 6 - Food and Beverage DivisiondDokument10 SeitenWorksheet Modul 6 - Food and Beverage DivisiondIAN WIDJAYANoch keine Bewertungen
- Chapter 14: Understanding Clutches and Their Operating PrinciplesDokument39 SeitenChapter 14: Understanding Clutches and Their Operating PrinciplespapipapiiNoch keine Bewertungen
- ALL-Q (Coenzyme Q10) Plus - PDSDokument3 SeitenALL-Q (Coenzyme Q10) Plus - PDSMarlon2370Noch keine Bewertungen
- Work Permit SystemDokument50 SeitenWork Permit SystemBin khammash and sons Co.Noch keine Bewertungen
- Adime NoteDokument2 SeitenAdime Noteapi-384503305100% (1)
- BE AMC-34-All Branches With IndexDokument223 SeitenBE AMC-34-All Branches With IndexSrikanth RangdalNoch keine Bewertungen
- Silo Surface Area CalculationDokument3 SeitenSilo Surface Area CalculationVIC EngineersNoch keine Bewertungen
- BBB BCP-15W Cycling ComputerDokument2 SeitenBBB BCP-15W Cycling ComputerDannyNoch keine Bewertungen
- Hypothalamus and Pituitary Gland: Master Regulators of HormonesDokument5 SeitenHypothalamus and Pituitary Gland: Master Regulators of HormonesLiv LeysonNoch keine Bewertungen
- Corrosion Management and Cathodic Protection GuideDokument52 SeitenCorrosion Management and Cathodic Protection GuideErol DAĞNoch keine Bewertungen
- 5.3 Resource - Allocation - of - Downlink - Heterogeneous - NOMA - Network - Based - On - Multi-User - With - Different - SpeedsDokument5 Seiten5.3 Resource - Allocation - of - Downlink - Heterogeneous - NOMA - Network - Based - On - Multi-User - With - Different - SpeedsmuradNoch keine Bewertungen
- Jss Academy Unit 1 BDokument13 SeitenJss Academy Unit 1 BbomtozorNoch keine Bewertungen
- Installation Manual flexES 798980.GB0Dokument64 SeitenInstallation Manual flexES 798980.GB0gius uddinNoch keine Bewertungen
- خصائص زحف السبيكة الثلاثية PDFDokument17 Seitenخصائص زحف السبيكة الثلاثية PDFEidelsayedNoch keine Bewertungen
- Central Sterile ServiceDokument75 SeitenCentral Sterile ServiceSUBHENDU SIKDAR100% (1)
- LPVDDokument12 SeitenLPVDPardha SaradhiNoch keine Bewertungen
- Care of Older Adults Notes PT 1Dokument5 SeitenCare of Older Adults Notes PT 1Edson John Demayo100% (1)
- q4_tleDokument65 Seitenq4_tleAngelica TaerNoch keine Bewertungen
- Clerical Exam Sample PaperDokument21 SeitenClerical Exam Sample PaperSarbjit Singh100% (1)
- Shortcuts and Quick Tips To Solve CAT MBA Quantitative Questions CAT, Entrance Exams, MBA - iHelpStudyDokument21 SeitenShortcuts and Quick Tips To Solve CAT MBA Quantitative Questions CAT, Entrance Exams, MBA - iHelpStudySofi DinendranNoch keine Bewertungen
- Skyrim Potions GuideDokument26 SeitenSkyrim Potions GuideMarkinhos RuviaroNoch keine Bewertungen
- Spence J Chemistry PHD 2018 PDFDokument383 SeitenSpence J Chemistry PHD 2018 PDFFLAVIANoch keine Bewertungen
- Group 9 Caught in Between Modern and Contemporary ArtDokument12 SeitenGroup 9 Caught in Between Modern and Contemporary Artlen lenNoch keine Bewertungen
- Facemasks Manufacturers DirectoryDokument54 SeitenFacemasks Manufacturers DirectoryAlex Kharchuk100% (1)
- Business Operations Group AssignmentDokument7 SeitenBusiness Operations Group Assignmentankit gangeleNoch keine Bewertungen
- Timing k4jDokument8 SeitenTiming k4jCryy73Noch keine Bewertungen
- Formation Damage ExamplesDokument89 SeitenFormation Damage ExamplesLaurensius Raymond SanjayaNoch keine Bewertungen
- M7 Lab: Sedimentary RocksDokument10 SeitenM7 Lab: Sedimentary RocksEssay NationNoch keine Bewertungen
- False Teachings TodayDokument7 SeitenFalse Teachings TodayobinwanneNoch keine Bewertungen
- New Holland Ec25 Mini ExcavatorDokument153 SeitenNew Holland Ec25 Mini ExcavatorJack StinerNoch keine Bewertungen