Beruflich Dokumente
Kultur Dokumente
Drugs Control
Hochgeladen von
Thirunavukkarasu BoopathyOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Drugs Control
Hochgeladen von
Thirunavukkarasu BoopathyCopyright:
Verfügbare Formate
Welcome Mr.
B Thirunavukkarasu
Sitemap | News | Contact us | My Account
Top Exciting Stories
Karnataka govt to have State Drugs & Cosmetic Rules by March end
(5/3/12)
Bangalore, 5 March: Karnataka government may soon come out with a set of dedicated State Drugs and Cosmetic Rules
under the Drugs & Cosmetics Act 1940. The state government had already formed an expert committee for the purpose
with the mandate to finalize these draft Rules shortly.
The three member committee is chaired by the former deputy drugs controller Rajashekariah. The other two members are
a Deputy Drugs Controller and Assistant Drugs controller from the Bangalore headquarters of the state drugs control
department.
"The government has been keen to frame the state specific rules within the frame work of the Central Act. These draft
rules will be first passed in the State assembly and then presented to the Union government for clearance. This is the first
of its kind exercise in the country," Dr BR Jagashetty, Drugs Controller, government of Karnataka told this reporter.
"We have been looking at methods to further strengthen the monitoring of quality of drugs and ethical trade practices in
the state. This led us to propose to the state government on the need for a separate State Drugs & Cosmetic Rules. The
state government has immediately taken cognizance of the same and called for the formation of a three -member
committee, he said.
This is probably the first initiative by a state government to go in for an exclusive set of state Drugs & Cosmetic Rules,
although the Maharashtra government had framed rules for fee structure for drug licensing. There are several areas which
the State Drugs &Cosmetic Rules will need to focus such as spurious drugs, not of standard quality medicines,
mushrooming of pharmacy outlets and the absence of a qualified pharmacist at the counters and the need to introduce
shifts for pharmacists at chemist shops besides review of various fee structures, said Dr Jagashetty.
The time frame set for the draft of the Karnataka Drugs & Cosmetics Rules is three months after which it would circulate
the same to seek comments. The industry and the pharmacy trade among others will be called for discussion to give a final
shape to the rules. The whole process is expected to take six months before the Rules would be tabled for clearance before
the state and Union governments, he said.
The key purpose of the Karnataka Drugs and Cosmetic Rules would be to bring in a strict and rigorous system of audit,
inspection and booking violators of the law. The creation of an efficient state regulatory system is viewed as an integral
component in public health.
The new rules are extremely urgent going by the changes taking place in the pharma and biotech industry. While it will
mandate the need for computerization as a benchmark to maintain transparency and efficiency, it is also expected to
include Good Distribution Practices (GDP) which will provide clear cut norms to the industry and trade on distribution,
recall and management of the date expired / discarded drugs within a given time frame. The State Drugs & Cosmetics
Rules could also be relevant for the country as it is also looking to define specific area for pharmacy outlets.
Incidentally Dr Jagashetty is also the chairperson of Committee for Amendments to the existing Drugs and Cosmetic Rules
1945 formed by government of India through the Drugs Control General of India (DCGI).
He has also been selected as member of sub-group on spurious and adulterated drugs which will support the Task Force
constituted to formulate a long term policy and strategy for strengthening of drug sector in the country. The three member
committee has also taken the required direction from the State drugs controller.
Pharmabiz
Other Stories
| (6/3/12)
Swiss Company Implicated In Supplying Fake Cancer Drug Sold In US (6/3/12)
Gujarat FDCA busts racket making IV stents without license near Ahmedabad (6/3/12)
Ephedrine worth Rs 1.25 crore seized in Tamil Nadu (5/3/12)
GoM to take final call on pricing formula of essential drugs under new policy (5/3/12)
India opposes pharma piracy pact by 10 nations (3/3/12)
Cipla slapped Rs 424 cr demand notices (3/3/12)
DrugsControl.org - http://drugscontrol.org/news.asp?id=7380
1 of 2 07-03-2012 12:21
55 fake drug dealers indicted in Beijing court (3/3/12)
| | (2/3/12)
Biosimilar products development gets a USFDA norms boost (2/3/12)
IPC to focus on developing reference substance for biological products (2/3/12)
Four children die in Kerala after administration of pentavalent vaccine (1/3/12)
PMO pushes for standard treatment protocols (1/3/12)
Fake Cancer Drug Contained a Variety of Compounds, But No Drugs (1/3/12)
US FDA announces safety changes in labeling for some cholesterol-lowering drugs (29/2/12)
Regulator for health sector; Law to be notified soon (29/2/12)
| | | (29/2/12)
US recalls India-made birth control pills (28/2/12)
Health ministry rejects DoPs recommendation on pricing of essential drugs (28/2/12)
Pharma Welfare body to protest against bias (27/2/12)
USP, IPC to agree on sharing information on chemical reference substances soon (27/2/12)
| (27/2/12)
Panel to examine death of baby given polio drops (27/2/12)
37 raids to bust fake drug racket (25/2/12)
DIA to organize 2-day conference on pharmacovigilance in Bengaluru on March 3 & 4 (25/2/12)
IDMA terminates all MoU's with AIOCD, advises members to follow provisions of Competition Act (25/2/12)
(25/2/12)
| | (24/2/12)
NPPA sends notices to Zydus, Beacon Pharma for violation of Para 18 of DPCO (24/2/12)
! (24/2/12)
! (24/2/12)
Claims of curing cancer to land Baba Ramdev in trouble (24/2/12)
2 held for selling fake drugs (23/2/12)
Pharmexcil to organise buyer-seller meet for medical device sector in Ahmedabad on Mar 23 (23/2/12)
Kerala drug control dept to introduce high tech facilities to provide better services (23/2/12)
Injection made from tap water (23/2/12)
US to import cancer drug from India (23/2/12)
Sex drugs for erectile dysfunction seized in Lambeth police raids (23/2/12)
Dr G N Singh appointed as new DCGI (22/2/12)
Low Levels of Omega-3 Fatty Acids May Cause Memory Problems (28/2/12)
Diabetes Drug Improves Glucose Control Without Increasing Risk of Hypoglycemia, Study Suggests (28/2/12)
New compound defeats drug-resistant bacteria (19/2/12)
More potent ways to design HIV drugs found (25/12/11)
Predicting Bad Drug Reactions Before They Happen (25/12/11)
Apixaban no more effective than enoxaparin in preventing VTE (ADOPT) (15/11/11)
Vaccine adverse events linked to virus disrupting ingredient (24/9/11)
IR Spectroscopy Defines Catalyzed Reactions (14/9/11)
EC develops tests for diethylhexyl phthalate adulteration of drugs, beverages (19/8/11)
Discovery opens new options for improving transfusions (17/7/11)
Off-label marketing of medicines in the US is rife but difficult to control (6/4/11)
News Search: Go!
Home || About us || Sitemap || News || Login || Quackwatch || Contact us || Query
Copyright DrugsControl.org - Jaipur, INDIA
DrugsControl.org - http://drugscontrol.org/news.asp?id=7380
2 of 2 07-03-2012 12:21
Das könnte Ihnen auch gefallen
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (121)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
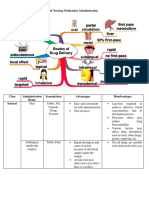
- Chapter 18 Common Drugs PDFDokument221 SeitenChapter 18 Common Drugs PDFlalallalaNoch keine Bewertungen
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- Anticancer DrugsDokument117 SeitenAnticancer DrugsKishore Chandra Korada100% (2)
- Different Routes of Nursing Medication AdministrationDokument13 SeitenDifferent Routes of Nursing Medication AdministrationRonica MendozaNoch keine Bewertungen
- ספרות מומלצתDokument6 Seitenספרות מומלצתbadu27Noch keine Bewertungen
- Status Epilepticus in Pregnancy A Literature Review and A ProtocolDokument12 SeitenStatus Epilepticus in Pregnancy A Literature Review and A Protocolmaria arenas de ita100% (1)
- Compilation of QuizzesDokument10 SeitenCompilation of QuizzesElesis samaNoch keine Bewertungen
- Liposomal Drug Delivery SystemDokument15 SeitenLiposomal Drug Delivery SystemsyeedNoch keine Bewertungen
- Wahyuningrum Indah Saraswati-01206321A - Tugas P.farprak - Penyimpanan ObatDokument50 SeitenWahyuningrum Indah Saraswati-01206321A - Tugas P.farprak - Penyimpanan ObatSaraswati Indah ArumNoch keine Bewertungen
- Dream Job AssignmentDokument3 SeitenDream Job Assignmentapi-354947009Noch keine Bewertungen
- Print OutDokument21 SeitenPrint OutHelen93Noch keine Bewertungen
- Palonosetron Hospira Epar Public Assessment Report - enDokument22 SeitenPalonosetron Hospira Epar Public Assessment Report - enDang Chi CongNoch keine Bewertungen
- Hot Topics in Heart Failure: New Data and Therapies That Are Changing The Treatment LandscapeDokument70 SeitenHot Topics in Heart Failure: New Data and Therapies That Are Changing The Treatment LandscapeSasmita DewiNoch keine Bewertungen
- DCO SyllabusDokument2 SeitenDCO SyllabusMrunal DixitNoch keine Bewertungen
- Structure Based Drug DesignDokument91 SeitenStructure Based Drug DesignMariamNoch keine Bewertungen
- Dopunska Lista Lijekova - Po Dijelovima - Web - Stupa Na Snagu - 23 - 06 - 2022Dokument256 SeitenDopunska Lista Lijekova - Po Dijelovima - Web - Stupa Na Snagu - 23 - 06 - 2022Alma FazlićNoch keine Bewertungen
- Assessing Drug Substances To Identify - Highly Hazardous - Compounds For Quality Risk Management ProgramsDokument1 SeiteAssessing Drug Substances To Identify - Highly Hazardous - Compounds For Quality Risk Management ProgramsMusab HashmiNoch keine Bewertungen
- PMC Buys Isochem - Generics No 334 20171205Dokument16 SeitenPMC Buys Isochem - Generics No 334 20171205Echo WackoNoch keine Bewertungen
- Pharmaco Vigilance Practice GuidelineDokument31 SeitenPharmaco Vigilance Practice GuidelineMEDNoch keine Bewertungen
- Enteric-Coated Alendronate Sodium Nanoliposomes - A Novel Formula To Overcome Barriers For The Treatment of OsteoporosisDokument6 SeitenEnteric-Coated Alendronate Sodium Nanoliposomes - A Novel Formula To Overcome Barriers For The Treatment of OsteoporosisRana Mohanna Al-abdaliNoch keine Bewertungen
- Prescription: Made by ShayanDokument14 SeitenPrescription: Made by ShayanShayan SiddiquiNoch keine Bewertungen
- The Introduction of Chemotherapy' Using Arsphenamine - The First Magic BulletDokument6 SeitenThe Introduction of Chemotherapy' Using Arsphenamine - The First Magic BulletdentsavvyNoch keine Bewertungen
- Acute Pyelonephritis Treatment & Management: Approach ConsiderationsDokument4 SeitenAcute Pyelonephritis Treatment & Management: Approach ConsiderationsPeter InocandoNoch keine Bewertungen
- Birmingham SquareDokument3 SeitenBirmingham SquarefoxypandaNoch keine Bewertungen
- Perspective in Pharmacy ReviewerDokument4 SeitenPerspective in Pharmacy ReviewerRosemarie OngNoch keine Bewertungen
- Chapter 4 Dangerous DrugsDokument14 SeitenChapter 4 Dangerous DrugsMary Joyce Anne ColloNoch keine Bewertungen
- Self-Medication Practices Among ResidentDokument4 SeitenSelf-Medication Practices Among ResidentSyed Shabbir HaiderNoch keine Bewertungen
- Drug StudyDokument6 SeitenDrug StudyGracie S. VergaraNoch keine Bewertungen
- Pedi Math Packet 2016-2017-1Dokument26 SeitenPedi Math Packet 2016-2017-1NursyNurseNoch keine Bewertungen
- Stok OpnameDokument262 SeitenStok OpnamenailifinaNoch keine Bewertungen
- Map PrepositionsDokument1 SeiteMap PrepositionsANGEL ALEXANDER HERNANDEZ CASTRONoch keine Bewertungen