Beruflich Dokumente
Kultur Dokumente
IV Pre Board
Hochgeladen von
prakhar153Originalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
IV Pre Board
Hochgeladen von
prakhar153Copyright:
Verfügbare Formate
IV Pre board 2011-12 Class- XII Subject: Chemistry Max.Marks: 70 General Instructions: 1. All questions are compulsory 2.
Question no. 1-8 are very short answer questions and carry 1 mark each. 3. Question no. 9-18 are short answer questions and carry 2 marks each. 4. Question no. 19-27 are also short answer questions and carry 3 marks each. 5. Question no. 28-30 are long answer questions and carry 5 marks each 6. Use log tables if necessary, use of calculators is not allowed. 1 Identify the reaction order for the following rate constant: k = 2.3 105 L mol1 s1 2 What is shape selective catalysis? 3 Elements of Group 16 generally show lower value of first ionisation enthalpy compared to the corresponding periods of group 15. Why? 4 Write the formula for the following coordination compounds: Tetraammineaquachloridocobalt(III) chloride 5 Which compound in each of the following pairs will react faster in SN2 reaction with OH? (CH3)3CCl or CH3Cl 6 Write IUPAC names of the following compound. Time : 3 hrs.
7 Arrange the following compounds in increasing order of their boiling points. CH3CHO, CH3CH2OH, CH3CH2CH3 8 What is deficiency disease caused by deficiency of vitamin A? 9 Give reasons for : (i)Basicity decreases in the order NH3 > PH3 > AsH3 > SbH3 > BiH3. (ii)Nitrogen is restricted to a maximum covalency of 4 but Phosphorus can make compound up to covalency 6 10 Complete the equations: (i)XeF6 + H2O
-
(ii) F2(g) + H2O(l)
11 Differentiate between (a) Order and molecularity (b) Elementary and complex reaction 12 The rate of a reaction quadruples when the temperature changes from 293 K to 313 K. Calculate the energy of activation of the reaction assuming that it does not change with temperature. 13 Atoms of element B form hcp lattice and those of the element A&C occupy 2/3rd of tetrahedral voids and 1/3 of the octahedral void respectively. What is the formula of the compound formed by the elements A,B andC ? 14 Niobium crystallises in face-centred cubic structure. If density is 8.55gcm3, calculate atomic radius of niobium using its atomic mass 93 u. 15 Write the Cell reaction, nernst equation and emf of the following cells at 298 K: Sn(s)|Sn2+(0.050 M)||H+(0.020 M)|H2(g) (1 bar)|Pt(s); ESn2+ /Sn =0.14V 16 What is spectrochemical series? Explain the difference between a weak field ligand and a strong field ligand by taking an example of a species having d configuration. 17 Complete the following reactions: (i) C6H5N2Cl + H3PO2 + H2O (ii)C6H5NH2 + H2SO4 (conc.) 18 Give plausible explanation for each of the following: (i) Ethylamine is soluble in water whereas aniline is not. (ii)Aliphatic amines are stronger bases than aromatic amines.
4
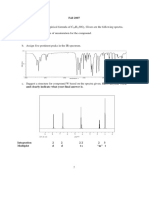
19 What do you mean by fuel cell? write half-cell reactions and overall cell reaction of H2 -O2 cell. Write also its advantages. 20 What is the difference between multimolecular and macromolecular colloids? Give one example of each. How are associated colloids different from these two types of colloids? 21 (a)Why copper matte is put in silica lined converter? (b)What is the role of cryolite in the metallurgy of aluminium? (c ) Zn and Cd are refined by distillation method. Why? 22 (a)The enthalpy of dissociation of F2 is lesser as compared to that of Cl2. Why? (b)Chlorine water on standing loses its yellow colour. Why? (b) Deduce the shape of BrF5. 23 (a)Explain the following with an example. (i) Reimer-Tiemann reaction. (ii) Williamson ether synthesis (b)Write the equation of the reaction of hydrogen iodide with methoxybenzene. 24 How will you bring about the following conversions? (i) Toluene to benzyl alcohol (ii) Propene to propyne (iii) Ethanol to ethyl fluoride 25 (i)How do you explain the amphoteric behaviour of amino acids? (ii)The two strands in DNA are not identical but are complementary. Explain. 26 Explain the following with one example: (i)Anti-histamine (ii)Analgesics (iii) broad spectrum antibiotics 27 Identify the monomer in the following polymer(i)Buna-N (ii) Neoprene (iii)
28 (a)Give simple chemical tests to distinguish between the following pairs of compounds. (iii) Phenol and Benzoic acid(v) Pentan -2-one and Pentan -3-one (vi)HCOOH and CH3COOH (b)Predict the products formed when cyclohexanecarbaldehyde reacts with following reagents. (i) PhMgBr and then H3O+ (ii) Semicarbazide and weak acid 29 Give reasons for: (i)Zr and Hf have very similar physical and chemical properties. (ii)The EM2+/M value for Ni is exceptionally more negative. (iii)The transition metals have high ability to form complexes. (b)Complete the following equations: (i)Cr2O72 + H+ + H2S (ii)MnO4 + S2O32 + H2O > (Basic medium)
30 (a)Distinguish between positive and negative deviation showing solutions on the basis of following points(i)solute-solvent interaction (ii) Vapour pressure of volatile components and total vapour pressure (iii) mixH (iv) mixV. Give one example of each. (b)Which gas will be more soluble in water He or H2 if KH for He and H2 are 144.97 and 69.16 kbar at 293K.
Das könnte Ihnen auch gefallen
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (73)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (120)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- Self Reliant Potter GLAZES You Can MakeDokument162 SeitenSelf Reliant Potter GLAZES You Can MakeStefan Van Cleemput100% (3)
- Slo Science Grade 7 Post Assessment 2016-2017Dokument15 SeitenSlo Science Grade 7 Post Assessment 2016-2017api-375209006Noch keine Bewertungen
- BIOB111 - Subject OutlineDokument7 SeitenBIOB111 - Subject OutlineMine CraftNoch keine Bewertungen
- Simple IUPAC NomenclatureDokument15 SeitenSimple IUPAC Nomenclatureapi-3757218100% (6)
- 1st Quarter Exam Science 7Dokument2 Seiten1st Quarter Exam Science 7Sherine Marianne IgnacioNoch keine Bewertungen
- Trial SPM SBP 2010 Chemistry Marking SchemeDokument18 SeitenTrial SPM SBP 2010 Chemistry Marking SchemeFain Sudais100% (1)
- 4541 SkemaDokument3 Seiten4541 Skemahairey947594Noch keine Bewertungen
- Chemistry - Periodic Table & ConfigurationDokument14 SeitenChemistry - Periodic Table & ConfigurationSajithKumarVariathNoch keine Bewertungen
- Skin. The Graph Shows The Action of The Fungal Enzyme at Different PH ValuesDokument14 SeitenSkin. The Graph Shows The Action of The Fungal Enzyme at Different PH ValuesLongNoch keine Bewertungen
- Pre Ph.D. Exam 2019-20: 9009-Ruhs PHD Exam (Pharmaceutical Chemistry) Question Paper With Answer Key Common SectionDokument20 SeitenPre Ph.D. Exam 2019-20: 9009-Ruhs PHD Exam (Pharmaceutical Chemistry) Question Paper With Answer Key Common SectionFarhadz Sailama BarahamaNoch keine Bewertungen
- Science 9 Q2 Week 3Dokument8 SeitenScience 9 Q2 Week 3harley_quinn11Noch keine Bewertungen
- Atomic Structures Formulas and NamesDokument4 SeitenAtomic Structures Formulas and NamesKyla Mari ValduezaNoch keine Bewertungen
- MCQ Exam#1Dokument3 SeitenMCQ Exam#1roxyNoch keine Bewertungen
- MSM-3 P Phases in Solids ( (Intro & Isomorphous System) PDFDokument15 SeitenMSM-3 P Phases in Solids ( (Intro & Isomorphous System) PDFShashank SinghNoch keine Bewertungen
- Chem in ContextDokument24 SeitenChem in ContextmumzeeNoch keine Bewertungen
- Test Bank For Introduction To Human Anatomy and Physiology 4th Edition by Pearl SolomonDokument6 SeitenTest Bank For Introduction To Human Anatomy and Physiology 4th Edition by Pearl Solomoncarwynquanh4tuozNoch keine Bewertungen
- Consumer Chemistry 1 - ReferenceDokument28 SeitenConsumer Chemistry 1 - ReferenceWhoami dela CruzNoch keine Bewertungen
- Soal latihanNMRDokument20 SeitenSoal latihanNMRAmalia Afiyanti25% (4)
- Ions and Ionic Bonding Pre-AICEDokument4 SeitenIons and Ionic Bonding Pre-AICEKelsey MarieNoch keine Bewertungen
- RPT Chemistry F4 2023Dokument9 SeitenRPT Chemistry F4 2023Ajlaa SudfiijNoch keine Bewertungen
- Organic Chemistry Structured Questions (Topical)Dokument28 SeitenOrganic Chemistry Structured Questions (Topical)Lee Jun Hui100% (1)
- 2types of Chemical ReactionsDokument13 Seiten2types of Chemical ReactionsLloyd Justine PoquitaNoch keine Bewertungen
- Science 7 - Diagnostic TestDokument9 SeitenScience 7 - Diagnostic TestMehara CaballeroNoch keine Bewertungen
- Chemical Bonding & ElectronegativityDokument4 SeitenChemical Bonding & ElectronegativityGwynethh EreseNoch keine Bewertungen
- Chemistry 6th EditionDokument22 SeitenChemistry 6th Editionhhonj0% (1)
- Physical Chemistry: Section 1Dokument956 SeitenPhysical Chemistry: Section 1Mitchel TayimoNoch keine Bewertungen
- # 1. Neet 2017 - Physics - Chapter 11 Kinetic TheoryDokument19 Seiten# 1. Neet 2017 - Physics - Chapter 11 Kinetic TheoryTamilaruviNoch keine Bewertungen
- CONCHEM Module 1 1Dokument22 SeitenCONCHEM Module 1 1Elynor Mataban100% (1)
- Test Bank Integrated Principles of Zoology 16th Edition Hickman Keen Larson RobertsDokument36 SeitenTest Bank Integrated Principles of Zoology 16th Edition Hickman Keen Larson Robertsyautiabacchusf4xsiy100% (31)
- Chemistry WorksheetDokument6 SeitenChemistry WorksheetOh Yoon AhNoch keine Bewertungen