Beruflich Dokumente
Kultur Dokumente
Anaphy of F&E
Hochgeladen von
Ino TiriOriginalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Anaphy of F&E
Hochgeladen von
Ino TiriCopyright:
Verfügbare Formate
ANATOMY AND PHYSIOLOGY
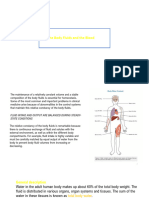
A. BODY FLUIDS- as primary body fluid, water is most important nutrient of life - Humans can survive for only a few days without water 1. FUNCTIONS OF WATER provide medium for transporting nutrients to cells, wastes from cells, and substances such as hormones, enzymes, blood platelets, and red and white blood cells facilitate cellular metabolism an d proper cellular chemical functioning act as solvent for electrolytes and nonelectrolytes help maintain normal body temperature facilitate digestion and promote elimination act as tissue lubricant BODY FLUID COMPARTMENTS a. Intracellular fluid (ICF) - within cells b. Extracellular fluid (ECF) - all fluid outside cells, including intravascular (plasma liquid component of blood) and interstitial (fluid that surrounds tissue cells and includes lymph) fluids total body water total amt of water in body expressed as % of body weight 3. VARIATIONS IN FLUID CONTENT - in a healthy person, total body water constitutes about 50 60% of bodys weight, depending on age, lean body mass, and sex - total body water differs by sex and persons amt of fat cells - fat cells contain little water while lean tissue is rich in water - women tend to have proportionally more body fat than men, they also have less body fluid than men - decreasing % body fluid in older people is related to increase in fat cells
2.
B.
ELECTROLYTES electrolyte substance capable of breaking into electrically charged ions when dissolved in a solution. Cations ions that develop a positive charge Anions ions that develop a negative charge 1. SODIUm (NA+) - chief electrolyte of ECF that moves easily between intravascular and interstitial spaces and moves across cell membranes by active transport - Influential in many chemical reactions in body, particularly nervous and muscle tissue cells - controls and regulates volume of body fluids; maintains water balance throughout the body - primary regulator of ECF volume and influences ICF - participates in generation and transmission of nerve impulses - essential electrolyte in sodium-potassium pump - normal extracellular concentration: 135 145 mEq/L
2.
POTASSIUM (K+) major cation of ICF working in reciprocal fashion with sodium (excessive intake of sodium results in excretion of potassium, vice versa) - chief regulator of cellular enzyme activity and cellular water content - plays vital role in such processes as transmission of electric impulses, particularly nerve, heart, skeletal, intestinal, and lung tissue; protein and carbohydrate metabolism, and cellular bldg. - adequate qty. usually in well-balanced diet - food sources include bananas, peaches, kiwi, figs, dates, apricots, oranges, prunes, melons, raisins, broccoli, potatoes, meat and dairy products - excreted primarily by kidneys, however, there are large amts in GI secretions and some in perspiration and saliva - normal range for serum: 3.5 5 mEq/L CALCIUM (CA+) most abundant electrolyte, with up to 99% of total found in iodized form of bones and teeth - close link between concentrations of calcium and phosphorus - necessary for nerve impulse transmission and blood clotting - catalyst for muscle contraction - needed for vitamin B12 absorption and its use by body cells - acts as catalyst for most cell chemical activities - necessary for strong bones and teeth - determines thickness and strength of cell membranes - adult avg. daily requirement about 1 g, higher amts. according to body wt. required for children and pregnant and lactating women - 1,500 mg/day recommended consumption for older adults, particularly postmenopausal women and men older than 65 - sources include milk, cheese, and dried beans, some present in meats and vegetables - excreted in urine, feces, bile, digestive secretions, and perspiration MAGNESIUM (MG2+) most cation found within body cells heart, bone, nerve, and muscle tissues - 2nd most important cation in ICF - important for metabolism of carbohydrates and proteins - important for many vital reactions involving enzymes - necessary for protein and DNA synthesis, DNA and RNA transcription, and translation of RNA - maintains normal intracellular levels of potassium - helps maintain electrical activity in nervous tissue and muscle membranes - adult daily avg. requirement about 18 30 mEq, with children requiring larger amts. - found in most foods, but especially in vegetables, nuts, fish, whole grains, peas and beans CHLORIDE (CI-) chief extracellular anion, found in blood, interstitial fluid, and lymph and in minute amounts in ICF - acts with sodium to maintain osmotic pressure in blood - plays a role in bodys acid-base balance - has important buffering action when oxygen and carbon dioxide exchange in red blood cells - essential for production of hydrochloric acid in gastric juices - found in foods high in sodium, dairy products, and meat - deficit leads to potassium deficit, and vice versa - normal serum levels: 95 105 mEq/L
3.
4.
5.
6.
BICARBONATE (HCO3-) anion that is major chemical base buffer within body - found in both ECF and ICF - essential for acid-base balance; bicarbonate and carbonic acid constitute bodys primary buffer system - losses possible via diarrhea, diuretics, and early renal insufficiency - excess possible via overingestion of acid neutralizers, such as sodium bicarbonate - normal levels range between 25 29 mEq/L 7. PHOSPHATE (PO4-) - major anion in body cells - buffer anion in both ICF and ECF - helps maintain bodys acid-base balance - involved in important chemical reactions in body (necessary for many B vitamins to be effective, helps promote nerve and muscle action, plays a role in carbohydrate metabolism) - important for cell division and transmission of hereditary traits - average daily requirements similar to calcium - found in most foods but especially in beef, pork, and dried peas and beans - inversely proportionate to calcium - - increase in one results in decrease of the other - normal range: 2.5 4.5 mEq/L
C.
FLUID AND ELECTROLYTE MOVEMENT - ECF provides nourishment to each body cell and receives cells waste products - these exchanges are essential to life
1. OSMOSIS through semipermeable membranes, water (pure solvent) is able to be transported through cell walls - major method of transporting body fluids - through osmosis, water passes from an area of lesser solute concentration to an area of greater solute concentration until equilibrium is established - the greater the concentration of the two solutions, the greater the osmotic pressure or drawing power of water osmolarity concentration of particles in a solution, or its pulling, power isotonic solution that has about the same concentration of particles (osmolarity) as plasma - remains in intravascular compartment w/out any net flow across semipermeable membrane hypertonic solution has greater osmolarity than plasma - water moves out of cells and is drawn into intravascular compartment, causing cells to shrink hypotonic solution has less osmolarity than plasma - solution in intravascular space moves out and into intracellular fluid, causing cells to swell and possibly burst 2. DIFFUSION tendency of solutes to move freely throughout a solute; coasting downhill - moves from area of higher concentration to an area of lower concentration until equilibrium is established - gases move by diffusion - oxygen and carbon dioxide exchange in the lungs alveoli and capillaries by diffusion 3. ACTIVE TRANSPORT process that requires energy for movement of substances through a cell membrane from an area of lesser solute concentration to an area of higher concentration - adenosine triphosphate makes it possible for certain substances to acquire energy needed - energy requirements for active transport are affected by characteristics of cell membrane, specific enzymes, and concentrations of ions
- pumping uphill - substances include amino acids, glucose (in certain places only - - kidneys, intestines), and ions of sodium, chloride, potassium, hydrogen, phosphate, calcium, and magnesium 4. FILTRATION passage of fluid through permeable membrane from area of high pressure to lower colloid osmotic pressure (oncotic pressure) certain substances, such as plasma proteins, which have high molecular weights on permeable membranes in the body. hydrostatic pressure force exerted by fluid against container wall - blood hydrostatic pressure is the pressure of plasma and blood cells in the capillaries-depends primarily on arterial blood pressure on arteriolar side of capillaries, and on venous blood pressure on venular side of capillaries - filtration pressure is the difference between colloid osmotic pressure and blood hydrostatic pressure - these pressures are important in understanding how fluid leaves arterioles, enters interstitial compartment, and eventually returns to venules - positive pressure in arterioles - - helping to force or filter fluids into interstitial space - negative pressure in venules - - helping fluid enter venules D. FLUID BALANCE - desirable amt of fluid intake and loss in adults ranges from 1500 3500 mL each 24 hrs., with most people averaging 2500 mL/day - individuals health state as well as balance between actual intake and loss must be considered - intake should normally be approx. balanced by output or fluid loss - may not always occur in a single 24-hr. period but should be achieved within 2 3 days FLUID SOURCES a. Ingested Liquids makes up largest amt of water intake - primarily regulated by thirst mechanism - stimulated by intracellular dehydration and decreased blood volume b. Water in Food 2nd largest source of water - depends on diet 1.
c. Water from Metabolic Oxidation occurs during metabolism of food substances, specifically, carbohydrates, fats, and proteins - source varies among different types of nutrients 2. FLUID LOSSES through kidneys as urine, intestinal tract in feces, and skin as perspiration (sensible losses) - insensible losses include ex. of invisible amt of water lost from skin constantly through evaporation and from the lungs exhaled as breaths - losses vary according to person and circumstances - any deviations from normal ranges for a balanced water intake and output should alert nurse to potential imbalances 3. HOMEOSTATIC MECHANISMS almost every organ and system in the body helps fluid homeostasis function automatically and effectively: kidneys (master chemists) selectively retain electrolytes and water and excrete wastes and excesses cardiovascular system is responsible for pumping and carrying nutrients and water
lungs regulate oxygen and carbon dioxide levels - - carbon dioxide is especially crucial in maintaining acid-base balance adrenal glands secrete aldosterone which helps body conserve sodium, helps save chloride and water, and causes potassium to be excreted thyroxine (from thyroid gland) increases blood flow leading to increased renal circulation, resulting in increased glomerular filtration and urinary output parathyroid glands secrete parathyroid hormone, regulating level of calcium in ECF GI tract absorbs water and nutrients nervous system (acting as switchboard to inhibit and stimulate mechanisms) regulates sodium and water intake and excretion
E. (H+)
ACID-BASE BALANCE - body fluids must maintain acid-base balance to sustain health and life - acidity or alkalinity of solution is determined by concentration of hydrogen ions acid substance containing hydrogen ions that can be liberated or released - strong acid dissociates (separates) completely and releases all hydrogen ions - weak acid releases only a small number of hydrogen ions base (alkali) substance that can accept or trap hydrogen ions - strong base binds/accepts H+ easily - weak base doesnt accept H+ easily
pH unit of measure used to describe acid-base balance - expression of hydrogen ion concentration and resulting acidity or alkalinity of a substance - scale ranges from 1 to 14 - neutral solution measures 7 (ex. pure water) - as hydrogen ions increase the solution becomes more acid, pH is less than 7 - as hydrogen ions decrease the solution becomes more alkaline, pH is greater than 7 - normal blood plasma is slightly alkaline and has a normal pH range of 7.35 7.45 - when blood plasma pH exceeds normal range in either direction, signs and symptoms of illness appear - if deviation goes unabated, death results acidosis condition characterized by excess hydrogen ions in ECF in which pH falls below 7.35 alkalosis lack of hydrogen ions and pH exceeds 7.45
Das könnte Ihnen auch gefallen
- VTH93-TWTJW-PTTC7-8CP8X-2XV82: Total Body WaterDokument5 SeitenVTH93-TWTJW-PTTC7-8CP8X-2XV82: Total Body WatermharjemmanNoch keine Bewertungen
- Fluid and ElectrolytesDokument17 SeitenFluid and Electrolytesdlneisha61100% (9)
- Fluid & Electrolyte PhysiologyDokument12 SeitenFluid & Electrolyte PhysiologyKristine Castillo100% (2)
- Fluids Electrolytes Acid Base DisordersDokument6 SeitenFluids Electrolytes Acid Base DisordersJerikaDolorPadilloPatricioNoch keine Bewertungen
- LECT 8 Fluids and Electrolyte Balance 2221Dokument32 SeitenLECT 8 Fluids and Electrolyte Balance 2221SaraNoch keine Bewertungen
- Fluids and ElectrolytesDokument69 SeitenFluids and ElectrolytesHarold DiasanaNoch keine Bewertungen
- Fluids and ElectrolytesDokument71 SeitenFluids and ElectrolytesHarold Castillo OrigenesNoch keine Bewertungen
- Fe ConceptDokument177 SeitenFe ConceptIvan MaximusNoch keine Bewertungen
- WandmDokument6 SeitenWandmJosiah Travis MontefalcoNoch keine Bewertungen
- Fluid, Electrolyte and Acid-Base Balance HomeostasisDokument28 SeitenFluid, Electrolyte and Acid-Base Balance HomeostasisHarley Justiniani Dela CruzNoch keine Bewertungen
- Mabes Fluid and ElectrolytesDokument9 SeitenMabes Fluid and ElectrolytesMabesNoch keine Bewertungen
- WATERDokument6 SeitenWATERAlthea Aine BalagotNoch keine Bewertungen
- Fluid and Electrolyte Imbalance and Nutritional ProblemDokument98 SeitenFluid and Electrolyte Imbalance and Nutritional ProblemPaul EbenezerNoch keine Bewertungen
- Nursing Care of Fluid, Electrolyte and Acid-Base ImbalancesDokument6 SeitenNursing Care of Fluid, Electrolyte and Acid-Base ImbalancesJames Kristopher RebayaNoch keine Bewertungen
- Water and Electrolyte BalanceDokument11 SeitenWater and Electrolyte BalanceVincent Maralit MaterialNoch keine Bewertungen
- Lecture 14. Water - Mineral Metabolism. Biochemistry of Kidneys and UrineDokument39 SeitenLecture 14. Water - Mineral Metabolism. Biochemistry of Kidneys and UrineВіталій Михайлович НечипорукNoch keine Bewertungen
- PHYSIOLOGY Lecture Notes Chapter 1Dokument9 SeitenPHYSIOLOGY Lecture Notes Chapter 1Aubrey Unique EvangelistaNoch keine Bewertungen
- Maintaining Fluid and Electrolyte BalanceDokument137 SeitenMaintaining Fluid and Electrolyte BalanceNano Baddour100% (1)
- Fluid and Electrolyte Imbalance GuideDokument20 SeitenFluid and Electrolyte Imbalance GuideesakkiammalNoch keine Bewertungen
- Body Fluids and BloodDokument83 SeitenBody Fluids and Bloodalthea jade villadongaNoch keine Bewertungen
- Electrolyte Imbalances and Fluid Volume DeficitsDokument49 SeitenElectrolyte Imbalances and Fluid Volume Deficitsuuuhbnb lplhgh100% (2)
- Revised Man As A Biological BeingDokument8 SeitenRevised Man As A Biological Beingapi-3832208Noch keine Bewertungen
- Nursing Care Plan of Client With Fluid and Electrolyte ImbalanceDokument28 SeitenNursing Care Plan of Client With Fluid and Electrolyte ImbalanceCj Aguilar50% (2)
- ACFrOgCEv6W8PZIOChX2ac TB PsVqkwB2LLEUA - nz2imEUDlJJqTMiew0fEYsQLz7I hoxDAHunIFV - PnnXqTDHrgpAsGRo2r9aPQF4UMBSoquxza - oenQBKFcGXpRcMCDKlMwgjULOtJeC9idDokument14 SeitenACFrOgCEv6W8PZIOChX2ac TB PsVqkwB2LLEUA - nz2imEUDlJJqTMiew0fEYsQLz7I hoxDAHunIFV - PnnXqTDHrgpAsGRo2r9aPQF4UMBSoquxza - oenQBKFcGXpRcMCDKlMwgjULOtJeC9idCenitserNoch keine Bewertungen
- Nursing Fluids and ElectrolytesDokument14 SeitenNursing Fluids and Electrolytesaga1028100% (18)
- Fluid, Electrolyte, and Acid-Base BalanceDokument41 SeitenFluid, Electrolyte, and Acid-Base BalanceRn nadeenNoch keine Bewertungen
- Fluid and Electrolyte BalanceDokument295 SeitenFluid and Electrolyte BalanceAnuchithra RadhakrishnanNoch keine Bewertungen
- MS Prelim ReviewerDokument5 SeitenMS Prelim Reviewerleslie_macasaetNoch keine Bewertungen
- Fluid and ElyctrolyteDokument49 SeitenFluid and Elyctrolyteuuuhbnb lplhghNoch keine Bewertungen
- Body Fluid Regulation and HomeostasisDokument27 SeitenBody Fluid Regulation and HomeostasisDesmy FadillahNoch keine Bewertungen
- TransDokument5 SeitenTranschayChay gapolNoch keine Bewertungen
- Fluid and ElectrolytesDokument9 SeitenFluid and Electrolytesfelicity tejadaNoch keine Bewertungen
- Fluids and ElectrolytesDokument131 SeitenFluids and ElectrolytesGianna j100% (1)
- Fluid and Electrolyte ConceptsDokument43 SeitenFluid and Electrolyte ConceptsQueenClerk VillarteNoch keine Bewertungen
- FLUID AND ELECTROLYTE HOMEOSTASISDokument21 SeitenFLUID AND ELECTROLYTE HOMEOSTASISMeryl RamosNoch keine Bewertungen
- FLUID, ELECTROLYTE and ACID-BASE BALANCE REGULATIONDokument61 SeitenFLUID, ELECTROLYTE and ACID-BASE BALANCE REGULATIONShanne PangpangdeoNoch keine Bewertungen
- Medsurg Lec FeDokument10 SeitenMedsurg Lec FeChelsea Faith SarandiNoch keine Bewertungen
- Fluid and Electrolyte ImbalanceDokument65 SeitenFluid and Electrolyte Imbalancesreenu100% (4)
- Fluid and electrolyte balance: Maintaining homeostasisDokument120 SeitenFluid and electrolyte balance: Maintaining homeostasisAlessandra MercadoNoch keine Bewertungen
- Reviewer Chapter 52 FluidsDokument8 SeitenReviewer Chapter 52 FluidsKeren GaciasNoch keine Bewertungen
- Transport Mechanism 2Dokument49 SeitenTransport Mechanism 2Jake Vincent LagapaNoch keine Bewertungen
- Fluid Electrolyte and AcidBase BalanceDokument33 SeitenFluid Electrolyte and AcidBase Balancemoncalshareen3Noch keine Bewertungen
- Output 1 Electrolytes - DraftDokument16 SeitenOutput 1 Electrolytes - Draftallanrnmanaloto100% (1)
- Final Water ElectrolytesDokument97 SeitenFinal Water ElectrolytesMonika shankarNoch keine Bewertungen
- 3.14 Chapter 3 Water and Electrolytes Balance and ImblanceDokument140 Seiten3.14 Chapter 3 Water and Electrolytes Balance and ImblanceShourav SarkarNoch keine Bewertungen
- Fluid, Electrolyte, and Acid-Base Balance GuideDokument29 SeitenFluid, Electrolyte, and Acid-Base Balance GuidemeriiNoch keine Bewertungen
- Bag - Isi Bu - Uut ElektrolitDokument13 SeitenBag - Isi Bu - Uut ElektrolitbianmanNoch keine Bewertungen
- Perioperative Fluid ManagementDokument123 SeitenPerioperative Fluid ManagementAnonymous 86gki5Noch keine Bewertungen
- The Water Prescription: For Health, Vitality, and RejuvenationVon EverandThe Water Prescription: For Health, Vitality, and RejuvenationBewertung: 5 von 5 Sternen5/5 (2)
- A-level Biology Revision: Cheeky Revision ShortcutsVon EverandA-level Biology Revision: Cheeky Revision ShortcutsBewertung: 5 von 5 Sternen5/5 (5)
- Metabolic Disorders and Critically Ill Patients: From Pathophysiology to TreatmentVon EverandMetabolic Disorders and Critically Ill Patients: From Pathophysiology to TreatmentCarole IchaiNoch keine Bewertungen
- Tyronn John Ramos Piamonte: ObjectiveDokument3 SeitenTyronn John Ramos Piamonte: ObjectiveIno TiriNoch keine Bewertungen
- Anaphy of F&EDokument5 SeitenAnaphy of F&EIno TiriNoch keine Bewertungen
- Tyronn John Ramos Piamonte: ObjectiveDokument3 SeitenTyronn John Ramos Piamonte: ObjectiveIno TiriNoch keine Bewertungen
- BPD Pathophysiology Linked to Mitochondrial DysfunctionDokument2 SeitenBPD Pathophysiology Linked to Mitochondrial DysfunctionIno TiriNoch keine Bewertungen
- AlzheimerDokument1 SeiteAlzheimerIno TiriNoch keine Bewertungen
- AlzheimerDokument1 SeiteAlzheimerIno TiriNoch keine Bewertungen
- Nursing Care Plan: Karla Female, 2 Y/o, CHDokument3 SeitenNursing Care Plan: Karla Female, 2 Y/o, CHIno TiriNoch keine Bewertungen
- AlzheimerDokument1 SeiteAlzheimerIno TiriNoch keine Bewertungen