Beruflich Dokumente
Kultur Dokumente
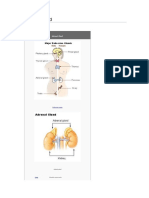
Adrenal Gland
Hochgeladen von
Yousf HandwaiOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Adrenal Gland
Hochgeladen von
Yousf HandwaiCopyright:
Verfügbare Formate
Adrenal Gland
Introduction:
Cortisol, adrenal androgens, & aldosterone (mineralocorticoid) are made in the cortex of the adrenal gland. The adrenal cortex has 3 zones (remember GFR): 1) The outer Zone Glomerulosa Mineralocorticoids 2) The middle Zone Fasciculata Cortisol 3) The inner Zone Reticularis androgens The chromaffin cells in the adrenal medulla manufacture mainly epinephrine A mnemonic is sweeter as you go in: outer = salt (mineralocorticoids), middle = sugar (glucocorticoids), inner = sex (adrenal androgens) CRH (corticortropin-releasing hormone) is secreted from the hypothalamus in response to a lowserum cortisol level, stress, & circadian rhythm. CRH cause the release of ACTH (stored in anterior pituitary) ACTH causes the adrenal gland to produce cortisol & androgens. It results in transient increase in mineralocorticoid production. ACTH has no effect on epinephrine production, which is under control of the nervous system. Cortisol stimulates lipolysis, the release of amino acids from the muscles, & gluconeogenesis by the liver, which uses the amino acid from the muscles, & causes centripetal fat distribution (central obesity). Cortisol also, inhibit all stages of inflammatory process. o Also affect the bone by decreasing the protein matrix & causing calciuria o Its immunosuppressive effect is on T cells & their associated cell-mediated immunity & delayed hypersensitivity. o Affect water balance by suppressing ADH & increasing GFR o 1 hour half life (to 90 minutes) In plasma < 5% of cortisol is free (no protein-bound) & therefore, physiologically active Only unbound free cortisol is filterable by the glomerulus; so urinary cortisol is always free cortisol reflection of plasma-free cortisol levels
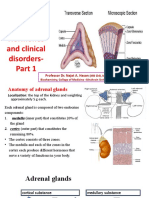
Anatomy:
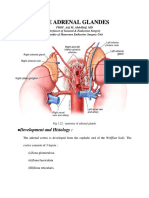
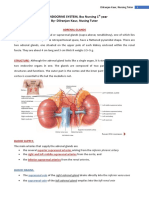
Adrenal (suparenal) gland: - Pair of glands lie near the superior poles of the kidney embedded in adipose tissue - Composed of cortex & medulla only gland Shape: - Right pyramidal - Left crescent half moon Relation: Right: - Superior pole of the kidney - Inferior& posterior to right liver lobe - Posterior to inferior vena cava - Anterior to right crus of diaphragm
Left: Upper med border of kidney Posterior to cardia of stomach Superior to pancreas Meddle to left crus of diaphragm
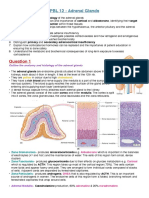
Structure: Fetal: - Gland larger - Composed of 3 layers: 2 cortex & 1 medulla Adult: Consists of : cortex & medulla Connective tissue capsule (rich in reticular fiber) & sends trabiculae into the gland Cortex consists of 3 parts: I. Zona Glmoerulosa II. Zona Fasiculata III. Zona Reticularis
Cortex: Zona Glmoerulosa: o 15% o Clumps of columnar cells o Round nucleus o Granules in cytoplasm rich in lipid droplets o Deep stain o Polyhydral o Mineralocorticoids (aldosterone) Zona Fasiculata: o 65% o Columns of cells o Surrounding capillaries o Lipid droplets, spongiocytes because of vacuolization during preparations o Glucocorticoids (cortisol & corticosterone) Zona Reticularis: o 7% o Irregular cords of cells; with pyknotic nuclei o Smaller cells; Anastamosing o Lipofuscin granules o Androgens Medulla: - Have double blood supply - Consists of 2 parts of: 1) Sympathetic ganglia: few & scattered, with no postganglionic neuron 2) Chromaphin cells - Neurons release their hormones into the blood Chromaphin cells: - Polyhydral - Clumps around capillaries with cords - Yellow - Larger & small cells 2
Larger: o 20% o Full of granules o Dense granule o Secrete noreepinephrine Small: o 80% o Secrete epinephrine Nerve supply: - Neurons without axons - Supply by the following nerves: o Celiac pleux o Renal pleux o X + phrenic Blood supply: - 3 artery from: o Aorta o Renal artery o Inferior phernic artery - 3 artery form the subcapsular pleux, under the capsule & form 2 pathways: 1) Then they supply cortex ( Glomerulosa, Fasiculata, Reticularis), capillarie contineu to medulla to supply it, collecting in adrenal vein to inferior vena cava 2) Capillaries go from pleuxes to medulla through cortex which supplying it, without branching - Capillaries of the medulla with capillaries of cortex form medullary veins, which join to constitute the adrenal or suparenal vein Development: We have the medulla & cortex (two endocrine glands in one). So, there will be two origins: *** The cortex from the celomic intermediate mesoderm. ( celomic intermediate: means body cavity . mesoderm:means peritoneum) # In week 4 : the celomic epithelium starts to develop & differentiate cells will go down & find the medulla & go around it so, the medulla develops before that-. #in week 8\9: there will be developed pituitary secreting ACTH & the suprarenal glands will secrete its hormones. *** The medulla from the neural crest cells. Note: the neural crest cells produce the peripheral nervous system.
Steroid synthesis:
Cholesterol is the precursor of all five classes of steroid hormones: glucocorticoids, mineralcorticoids, androgens, estrogens & progestins. Because of hydrophobicity; they cross the cell membrane & bind specific receptors in either the cytoplasm or nucleus. 3
The bound receptor then binds to DNA & regulate gene transcription So steroid & vitamin D: o Lipid soluble o Receptor location is intracellular Cholesterol is 1st acted upon by P450scc & 6-carbon units, forming the 21 carbon steroid, pregnenolone. (ACTH stimulation) RATE LIMITING STEP, for synthesis of all steroid hormone Pregnenolone is commoner precursor for all the steroid hormones Progesterone is the first steroid hormone formed from pregnenolone by 3-hydroxysteriod dehydrogenase (not member of P450) OR other enzyme member of P450 Progesterone is converted to other hormones (they reaction effected by hydroxylation) cholesterol is converted to progesterone in the first 2 steps of synthesis of all steroid hormone cytochrome P450SCC side-chain cleavage enzyme is located in the mitochondrial inner membrane & remove 6 carbones from the side chain of cholesterol , forming pregnenolone, which has 21 carbons. Cholesterol side chain cleavage (CSCC) is the rate limiting reaction in adrenal hormone synthesis Pregnenolone is formed in inner membrane of mitochondria & then shuttled between ER & mitochondria for further transformation to synthesis a derivative steroid hormone The next step is conversion of pregnenolone to progesterone, is catalyzed by 3 -hydroxysteriod dehydrogenase not member of cytochrome P450 Newly synthesized steroid hormone are rapidly secreted from the cell (little storage!) Following secretion all steroid binds to plasma proteins (different %) Serum albumin serves as non-specific carrier for steroid hormone; but there are specific carrier Steroid hormone are typically eliminated by inactivating metabolic transformations & excretion in urine or bile Cholesterol used for steroid hormone synthesis is either synthesized in the tissue from acetyl CoA, extracted from intracellular cholesterol ester pools, or taken up by the cell in the form of cholesterol-containing protein by internalized by the LDL-receptor
Structure: - They are derivatives from cholesterol, which composed from 27 carbon, but products: Glucocorticoids & progestins 21 carbon Androgens 19 carbon Estrogens 18 carbon - Specific complement enzymes present in the cells of an organ determine with hormones the organ can synthesize - General structure feature of steroid hormone: 1) Intact 4 ring system (except vitamin D1, where the B-ring has been opened) 2) Often contain hydroxyl side chains sterols2 o Hydroxyl groups called if oriented above the plane o Denoted if they oriented below the plane General notes about synthesis of glucocorticoids & Mineralocorticoids: Glucocorticoids: o Oxidative reactions that lead to the synthesis & secretion of cortisol are stimulated by adrenal corticotrophic hormone (ACTH) Mineralocorticoids: o Such as aldosterone, synthesized in adrenal cortex & secreted in response to angiotensin II or III, rising potassium (K+) levels in blood, & hyponatremia (low levels of sodium ions in the blood)
1 2
is a steroid hormone
Cholesterol
o Aldosterone stimulates Na+ reuptake in the kidney, sweat glands, salivary glands, with resultant increase in ECF volume & eventually in blood pressure Synthesis of Cortisol: - Biosynthetic pathway occurs in middle layer of the adrenal cortex called the Zona fasciculate - Cholesterol is synthesized from acetyl CoA or derived from LDL, which is endocytosed & digested by lysosomal enzymes. - Cholesterol is stored in cells of the adrenal cortex as cholesterol esters. - ACTH signals the cell to convert cholesterol to cortisol. - Free cholesterol is transported by intracellular carrier protein to the inner mitochondrial membrane of cells, where the side chain is cleaved to form pregnenolone. - Pregnenolone returns to the cytosol, where it forms progesterone. C-21 steroid hormone - In the membranes of the ER, the enzyme P450C17 catalyze the hydroxylation of C17 of progesterone or pregnenolone & catalyze the cleavage of the 2-carbon side chain of these compounds at C17 a hydroxyl group is added to carbon 17 - Those that keep their side chain are precursors of cortisol (C21), whereas those from which side chain was cleaved (C19) are precursors of androgens & estrogens - The 17-hydroxylation of progesterone yields the 17--hydroxyprogesterone, which, along with progesterone is transported to SER - 17-hydroxypregnenolone will be converted into 17-hydroxyprogesterone by two enzymes, they are 3-hydroxysteroid DH and D5-D4 isomerase. - oxidation on carbon3 so the hydroxyl group will be converted into keto-group - There the membrane bound 21--hydroxylase (P450C21) enzyme catalyzes the hydroxylation of C21 of 17--hydroxyprogesterone to form 11-deoxycortisol - First step of cortisol synthesis requires transport of 11-deoxycortisol back to the inner membrane of mitochondria, where 11--hydroxylase (P450C11) form cortisol Synthesis of corticosterone: - Conversion of cholesterol to progesterone o Direct oxidation of pregnenolone by 3-hydroxysteriod DH & D5-D4 isomerase - Progesterone is then hydroxylated at C21, a reaction catalyzed by P450C21, to yield deoxycorticosterone [DOC] - The P450C11 enzyme system then catalyzes the reaction that converts DOC to corticosterone. Aldosterone: - Oxidation of corticoterone to 18-hydroxycorticosterone, which is oxidized to aldosterone - Primary stimulus for aldosterone production is angiotensin II, although hyperkalemia or hyponatremia may directly stimulate aldosterone synthesis as well Notes: Cholesterol has to pass into the mitochondria with the help of protein called STAR steroidogenic Acute Regulatory protein that induced by ACTH First step & last step will take place in the mitochodria, & rest in the ER ACTH works of the 2nd messengers Also ACTH activate genets that give us different hydroxykase enzymes
MECHANISM OF ACTION : - Interaction with receptor promote DNA transcription to produce mRNA, & mRNA will translate to protein -
Physiology of adrenal gland:
5
All are steroid derived from cholesterol 3 important hormones are secreted by adrenal cortex: o Glucocorticoids cortisol increase blood glucose concentration, & affect protein & fat metabolism o Mineralocorticoids aldosterone affect electrolyte of ECF, Na, K o Androgenic hormones - The above hormones are secreted by different areas of the adrenal cortex: 1) The Zona glumerulosa o Synthesis aldosterone, because it contains the enzyme aldosterone synthase o Secretion controlled by extracellular fluid concentrations of angiottensin II & K+ 2) The Zona fasiculata o Secretes glucocorticoids, cortisol & corticosterone o Also small amount of adrenal androgens & estrogens o Secretion controlled by hypothalamic-pituitary axis via adrenocorticotropic hormone (ACTH) 3) The Zona Reticularis: o Secrete adrenal-androgens dehydroepiandrosterone (DHEA) & small amounts of estrogens & glucocorticoids o Secretion controlled by ACTH, & cortical androgen-stimulating hormone, released from the pituitary gland - The adrenal hormone are degraded mainly in the liver & conjugated to glucuronic acid, & to sulfates. - 25% of conjugates are excreted in bile & then in feces, remaining excreted in the urine Hormone & plasma protein: - 90-95% of cortisol in plasma binds to plasma proteins; especially to globulin called cortisol-binding globulin or transcortin & to albumin - Because of binding, its slows the elimination of cortisol from the plasma result in long half-life from 60-90 minutes - Normal concentration of aldosterone in blood is 6 ng/100 ml - The normal concentration of cortisol is 12 g/100 ml Regulation of secretion: - Angiotensin II increase output of aldosterone hypertrophy Zona glumerulosa - ACTH increase secretion of cortisol & adrenal androgens hypetrophy of zona fasiculata & zona reticularis Mineralocotricoids Aldosterone Function of mineralocorticods-aldosterone: Promotes sodium & water retention o Aldosterone act primarily on the kidney o It increase sodium reabsorption in the tubule, as a result body retain sodium o Secondary to Na retention, water is also retained Increase renal loss of potassium (hyperkelemia) Aldosterone dysfunction: Aldosterone deficiency causes severe renal Na-Cl wasting & hyperkalemia increase in K cocnetration Total loss result in death within 3 days to 2 weeks Excess aldosterone increase extracellular fluid volume & atrial pressure Also excess aldosterone causes hypokalemia & muscle weakness too little aldosterone casue hyperkalemia & cardiac toxicity increased serum K concentration 6
weakness of heart contraction development of arrhythmia heart failure excess aldosterone increase tubular hydrogen ion secretion & cause mild alkalosis aldosterone stimulates Na & K transport in sweat glands, salivary glands & intestinal epithelial cells
Excess-aldosterone secretion: Conns syndrome Excess aldosterone secretion leads to sodium retention & excess potassium loss Sodium retention leads to water retention Sodium & water retention may lead to hypertension Along with K excretion, aldosterone also increases renal hydrogen ion excretion Thats leads to alkalosis Deficient aldosterone secretion: Excess Na & water loss may leads to hypotension Reduced K excretion leads to hyperkalemia Reduced hydrogen ion excretion leads to acidosis Regulation of aldosterone secretion: - primarily regulated by 2 factors: plasma K & rennin-angiotensin system - other factors are: 1) concentration of ECF electrolytes 2) extracellular fluid volume 3) blood volume 4) blood pressure 5) increased & decreased potassium ion cocnetration 6) increased levels of angiotensin II 7) increased or decreased sodium ion concentration 8) ACTH (necessary for aldosterone secretion!) So regulation: 1) Direct stimulation of the adrenal cortex by a rise in plasma K+ concentration 2) Activation of rennin-angiotensin-aldosterone system by factors related to a reduction in Na+ & fall in blood pressure Notes: - Addisons disease, result from failure of adrenal cortex to produce adrenocortical hormones - Sing & symptoms: 1) Decrease in blood & body fluids 2) Hyponatremia sodium low level aldosterone 3) Hyerkalemia increase in K aldosterone 4) Mild acidosis---- deficiency of aldosterone 5) Decrease in blood volume --- loss of Na---- loss in water 6) Increased blood cells concentration ---- decrease in body fluids 7) Decrease in cardiac output = patient die in shock Glucocorticoids: - 95% of glucocorticoids function result from secretion of cortisol & corticosterone Cortisol: Action of cortisol: 1. Carbohydrate metabolism: o Stimulate gluconeogensis (production of glucose from amino acids) 6 10 folds, result from increasing enzymes required (by cortisol!) & mobilization of amino acids from extrahepatic tissue (muscle!) o increase glucoenogensis increase glycogen storage in the liver cells 7
o When cortisol is present, Glucagon & adrenaline (epinephrine) are most effective in producing glycogenolysis in muscles useful in stress. o Cortisol reduces peripheral utilization of glucose in most tissue except brain & heart. o Above effects (namely increase gluconeogenesis & moderate reduction in rate of glucose utilization) cause increase in blood glucose & in turn stimulate secretion in insulin, causing adrenal diabetes. 2. Protein metabolism: o At physiological levels, cortisol promotes protein synthesis o High level of cortisol (seen in stress), promotes protein breakdown & synthesis (catabolism) everywhere in the body except in the liver o Amino acid mobilized by protein breakdown are converted into glucose in the liver & utilized to meet requirements of stress. 3. Fat metabolism: o Cortisol promotes lipolysis directly & indirectly by facilitating the action of adrenaline & growth hormones (useful in stress) 4. Muscle contraction: o Increase the forces of skeletal muscle contraction (useful in stress!) 5. Role in stress o Physical & mental stress stimulate release of ACTH, consequently increase secretion of glucocorticoids 6. Anti-inflammatory effects o Blocks early stage of inflammation process o Cause rapid resolution of inflammation & increase rapidity of healing. o Stabilizes the lysosomal membranes, decreased permeability of capillaries (prevents loss of plasma) o Decrease both migration of white blood cells into the inflamed area & phagocytosis of the damaged cells 7. Immunosuppressive effects: o Decrease lymphocytes reproduction o Attenuates fever mainly because it reduces the release of IL-1 from WBC Other effects of cortisol: 1) Blocks the inflammatory response to allergic reaction; prevents shock or death in anaphylaxis 2) Effects on blood cells & immunity in infection deisease; decreasing number of esinophils & lymphocytes in the blood Regulation of ACTH: - Cortisol secretion is directly regulated by ACTH (-ve feedback), involving hypothalamus & anterior pituitary - Glucocorticoid (cortisol) secretion is stimulated by ACTH (adrenocorticotropic hormone), which is secreted by the anterior pituitary - ACTH secretion is stimulated by corticotropin releasing hormone (CRH), which is secreted by the hypothalamus & is delivered to the anterior pituitary by the hypothalamo-hypophyseal portal vessels chain called hypothalamus-pituitary adrenal axis - Secretion of ACTH & CRH is subject to ve feedback inhibition Notes: - Cortisol secretion secreted in Circadian rhythm, thats mean that secretory rate of CRF, ACTH & corticol are high in the early morning, but low in the late evening change with sleeping pattern - Synthesis & secretion of ACTH in association with melanocyte-stimulating hormone, lipoprotein & endocrine, from the anterior pituitary Excess Glucocorticoid secretion Cushings Syndrome: - Excess of glucocorticoids leads to a fat round face & fat deposition in the abdomen but the limbs remain thin. - Hair growth at all vulnerable areas of the body is stimulated - Muscles are wasted & bones develops osteoporosis due to protein catabolism. 8
Blood sugar may be raised primarily due to gluconeogensis Resistance to infection is impaired due to immunosuppression The blood pressure raised Na & water retention occurs due to the weak mineralocorticoid activity of glucocorticoids, which becomes significant when secretion is excessive
Deficient Glucocorticoid secretion Addisons Disease - Deficiency of glucocorticoids leads to loss of appetite, loss of weight, anemia & weakness. - It impairs the capacity of the body to cope up with stress. - Blood pressure may be low - Body fluids may be depleted of Na & water - Blood tests show lymphopenia & eosinopenia - If the deficiency is due to a defect in adrenal cortex itself, -ve feedback leads to elevated ACTH levels. - Elevated ACTH levels leads to pigmentation of the skin & mucous membrane. Adrenal Medulla: - Adrenal medulla is composed of modified postganglionic sympathetic neurons - Postganglionic in the adrenal medulla dose not posses axonal fibers that terminate on effector organs, instead within the adrenal medulla release their chemical transmitter directly into circulation upon stimulation by preganglionic fiber - Preganglionic fibers, terminate on the chromaffin cells of the adrenal medulla - Adrenal medulla secretes catecholamines, epinephrine & noreepinephrein (derived from tyrosine) - Mostly secreted is epinephrine - So chromaffin cells are equivalent to postganglionic neurons. - Adrenal medulla activated whenever sympathetic nervous system is activated. (sympathetic release norepinephrien where medulla epinephrine) - Secretion of catecholamines is stimulated by acetylcholine release from preganglionic sympathetic fibers innervating the medulla Action of Epi- & nore-epi: 1) Increase rate & force contraction of heart epinephrine effect 2) Norepinephrein cause vascoconstriction 3) Dilation of bronchioles 4) Stimulation of lipolysis in fat cells 5) Epinephrine increase metabolic rate 6) Dilation of pupils 7) Inhibit GI secretion & motility 8) Increase blood glucose & fatty acids 9) Decrease blood flow to GI organs, kidneys, & skin 10) Increase blood flow to heart, brain, & skeletal muscles Regulation of catecholamine secretion: 1) Exercises 2) Hypoglycemia 3) Hemorrhages 4) Emotional distress 5) Trauma - In general regulation is linked up with regulation of ANS.
Adrenal Gland Disease:
9
Disease of the adrenal gland could be: 1) Hyperfunction 2) Hypofunction 3) Tumors
Adrenocortical Hyperfunction: - 3 syndrome associated with Hyperfunction:
1 2 3
Cushings syndrome & Cushings disease Conns syndrome & Hyperaldosteronism Adrenogenital syndrome
1. Cushings Syndrome - Occurs when there is excessive adrenal glucocorticoid production. - Cause can be classified in Endogenous causes & Exogenous causes: A. Endogenous causes: I. ACTH Adrenocorticotropic hormone secreting pituitary microadenoma, few macroadenomas OR hyperplasis Cushings disease 2 to increased pituitary ACTH production is called Cushing disease II. Adrenal tumor or hyperplasia III. Paraneoplastic syndrome B. Exogenous causes: I. Steroid therapy - Tests used are: Level cortisol in plasma Excretion of 17-hydroxy steroids in urine Diurnal pattern Level of ACTH Dexamethasone suppression test If it is Cushing disease, ACTH will be elevated, causing an increase in DHEA (& urinary 17ketosteriods). Therefore the patient can present with androgen excess signs (hirsutism) If there is an adrenal adenoma producing the cortisol, ACTH will be low & therefore DHEA will be low Tests in adrenal carcinoma are the same as with adenoma, except the DHEA is elevated because adrenal carcinoma usually produce extra androgens In all Cushing syndromes, except plasma cortisol & therefore, urinary free cortisol to be elevated Morphology of adrenals in Cushings syndrome: o Depends on the cause Exogenous increase in glucocorticoids ACTH Bilateal atrophy of adrenals Endogenous: a. Adrenal adenoma or carcinoma atrophy of adjacent & contralateral adrenal b. Secondary to ACTH secreting adenoma bilateral diffuse or nodular hyperplasia c. Primary adrenal nodular hyperplasia Pituitary in all forms of Cushings syndrome shows alteration in ACTH producing cells:
10
Granular basophilic cells show lighter homogenized cytoplasm due to accumulation of intermediate keratin filaments in cytoplasm, called Crookes Hyaline change Clinical features of cushing syndrome: o Central obesity o Moon face o Hypertension o Hirsutism o Menstrual disturbances o Diabetes o Osteoporosis o Increase risk of infections o Pigmentation of skin
1 2 3
Cushings syndrome & Cushings disease Conns syndrome & Hyperaldosteronism Adrenogenital syndrome
2. Hyperaldosteronism: - Excess of aldosterone cause Na+ sodium retension & potassium excretion, resulting in hypertension & hypokalemia - Could be primary or secondary - Primary aldosteronism Conns syndrome: o Most often (80%) due to a benign adenoma in zona glomerulosa F:M 2:1 Single or multiple o 15% due to primary adrenal hyperplasia o Carcinoma is rare o Adjacent adrenal cortex is not atrophic o aldosterone Na+ retention & K+ excretion BP, Hypokalemia, Renin o Correctable cause of hypertension - Secondary aldosteronism: o Compensatory reaction related to decrease in cardiac output decrease renal perfusion o Decrease renal blood flow activates the rennin-angiotensin-aldosterone system aldosterone o Plasma rennin activity is increase ; differentiate from primary
1 2 3
Cushings syndrome & Cushings disease Conns syndrome & Hyperaldosteronism Adrenogenital syndrome
11
3. Adrenogenital syndrome: - Commonly known as congenital adrenal hyperplasia, group of conditions - There is disorders of steroid hormone production in the adrenal glands leading to deficiency of cortisol, the pituitary, sensing the deficiency, secrets massive amounts of the stimulating hormone corticotropin to bring the cortisol levels up to normal. This hormone in turn causes the adrenal glands to overproduce certain intermediary hormones which have testosterone-like effects on the fetus & child, leading to Virilizing syndromes Virilizing syndrome: - Virilization means that the clitoris of girls is enlarged & may resembles the male penis, to the point that the sex of child is questioned or mistaken. - Virilizing syndrome: o Include several phases, not just adrenal o Caused by: Primary gonadal disorders (ovaries or tests hormone inducing virilizing) Adrenocortical neoplasm Congential adrenal hyperplasia = Adrenogenital syndrome - Congenital adrenal hyperplasia is casued by enzyme defect in cortisol synthesis (21 hydroxylase) No cortisol ACTH androgenic steroids - The virilizing tumor are more likely to be malignant - Virilizing tumor is not common - Virilization may occure in children & cause precocious puberty (it boys at age of 8) & ambiguous genitalia (in girls, enlargement of clitoris) - Patient have risk for acute adrenocortical insufficiency Morphology in All adrenal tumors: o Encapsulated, yellow o Size variable o Most incidental non-functioning tumors (may be functioning) o Maliganat tumors with necrosis, hemorrhage (> 300 gms) o Usually larger, more aggressive in adults o Benign or malignant may show same appearance of uniform or slightly plemorphic cells o May be esinophilic or clear o Local invasion o Presence of metastases differentiate benign from malignant tumors.
12
Das könnte Ihnen auch gefallen
- Adrenal Gland: Structure, Hormones and Clinical Disorders-: Professor Dr. Najat A. HasanDokument18 SeitenAdrenal Gland: Structure, Hormones and Clinical Disorders-: Professor Dr. Najat A. HasanDrFarah Emad Ali100% (1)
- 5 Adrenal GlandDokument8 Seiten5 Adrenal Glandapi-3706483Noch keine Bewertungen
- Endocrine Metabolism: Adrenal GlandsDokument2 SeitenEndocrine Metabolism: Adrenal GlandsEcaterina Borovic-PavlovschiNoch keine Bewertungen
- Adrenal Cortex HormonesDokument21 SeitenAdrenal Cortex HormonesAaa JjjjNoch keine Bewertungen
- 6-Adrenocortical Hormones PancreaseDokument47 Seiten6-Adrenocortical Hormones Pancreasetmqt2fbnzgNoch keine Bewertungen
- 8 Hormones of Adrenal GlandDokument27 Seiten8 Hormones of Adrenal GlandRana AbdullahNoch keine Bewertungen
- Adrenal GlandDokument50 SeitenAdrenal GlandAnita YadavNoch keine Bewertungen
- Adrenocortical HormonesDokument46 SeitenAdrenocortical HormoneschayChay gapolNoch keine Bewertungen
- Chapter 79. Adrenal GlandsDokument42 SeitenChapter 79. Adrenal GlandsJeremy IrvanNoch keine Bewertungen
- Adrenal GlandDokument36 SeitenAdrenal Glandpranutan739Noch keine Bewertungen
- Chap3 The Adrenal GlandDokument20 SeitenChap3 The Adrenal GlandSharon GabrielNoch keine Bewertungen
- 17 Adrenal GlandDokument33 Seiten17 Adrenal GlandPande Indra PremanaNoch keine Bewertungen
- Steroid Hormone-SynthesisDokument40 SeitenSteroid Hormone-SynthesisAbubakar SuleimanNoch keine Bewertungen
- 3-Adrenal Glands and Its Hormones-MWDokument103 Seiten3-Adrenal Glands and Its Hormones-MWAbditsion DhiisaniiNoch keine Bewertungen
- The Adrenal GlandesDokument19 SeitenThe Adrenal GlandesamrsameerNoch keine Bewertungen
- 1.adrenal Gland AnatomyDokument60 Seiten1.adrenal Gland AnatomyAstha ShresthaNoch keine Bewertungen
- PBL 12 - Adrenal GlandsDokument7 SeitenPBL 12 - Adrenal GlandsKrishna OochitNoch keine Bewertungen
- B.SC - Hons. Biotechnology 4th Sem Dr. Arvind ShuklaDokument7 SeitenB.SC - Hons. Biotechnology 4th Sem Dr. Arvind Shuklamain.manishapatelNoch keine Bewertungen
- Insufisiensi Adrenal (Dr. Makbul)Dokument52 SeitenInsufisiensi Adrenal (Dr. Makbul)Rahmat AzimiNoch keine Bewertungen
- The Endocrine SystemDokument65 SeitenThe Endocrine SystemleanavillNoch keine Bewertungen
- Adrenal Gland - DR CokerDokument59 SeitenAdrenal Gland - DR CokerIshaqNoch keine Bewertungen
- Adrenal Glands 2018 - 2019Dokument32 SeitenAdrenal Glands 2018 - 2019Bianca BiaNoch keine Bewertungen
- RenalDokument23 SeitenRenalSabbir AhmedNoch keine Bewertungen
- The Adrenal Gland: Key ConceptsDokument26 SeitenThe Adrenal Gland: Key ConceptsZIA QURESHINoch keine Bewertungen
- Adrenal Gland by DR Rajnee Ist PartDokument29 SeitenAdrenal Gland by DR Rajnee Ist Part9460106212Noch keine Bewertungen
- Biosynthesis of Cortisol Ics-2Dokument3 SeitenBiosynthesis of Cortisol Ics-2Kate Alyssa CatonNoch keine Bewertungen
- HormonesDokument124 SeitenHormonesMr T KillworthNoch keine Bewertungen
- 01 EndocrineDokument40 Seiten01 EndocrineMavi SaldevarNoch keine Bewertungen
- Functional Anatomy of The Adrenal GlandDokument9 SeitenFunctional Anatomy of The Adrenal GlandPaula SchaeferNoch keine Bewertungen
- Endo 3 Notes PDFDokument9 SeitenEndo 3 Notes PDFDilNoch keine Bewertungen
- Endicronology Anatomy 3Dokument4 SeitenEndicronology Anatomy 3shahab shamsiNoch keine Bewertungen
- 10 The Adrenal Gland Aldosterone2017 1Dokument27 Seiten10 The Adrenal Gland Aldosterone2017 1Hassan AhmedNoch keine Bewertungen
- Adrenal GlandDokument46 SeitenAdrenal GlandhSANNoch keine Bewertungen
- 5adrenal GlandDokument6 Seiten5adrenal GlandZiedTrikiNoch keine Bewertungen
- Steroid HormDokument40 SeitenSteroid HormleNoch keine Bewertungen
- Adrenal & KortisolDokument47 SeitenAdrenal & KortisolPrisca AngelinaNoch keine Bewertungen
- Online Practice Tests, Live Classes, Tutoring, Study Guides Q&A, Premium Content and MoreDokument17 SeitenOnline Practice Tests, Live Classes, Tutoring, Study Guides Q&A, Premium Content and MoreYoAmoNYCNoch keine Bewertungen
- Online Practice Tests, Live Classes, Tutoring, Study Guides Q&A, Premium Content and MoreDokument17 SeitenOnline Practice Tests, Live Classes, Tutoring, Study Guides Q&A, Premium Content and MoreabctutorNoch keine Bewertungen
- Online Practice Tests, Live Classes, Tutoring, Study Guides Q&A, Premium Content and MoreDokument17 SeitenOnline Practice Tests, Live Classes, Tutoring, Study Guides Q&A, Premium Content and MoreabctutorNoch keine Bewertungen
- Endo Adrenal ElhDokument26 SeitenEndo Adrenal Elhodiodi57Noch keine Bewertungen
- Adrenal GlandDokument49 SeitenAdrenal GlandRaphael AnajeNoch keine Bewertungen
- Chapter 8 The Endocrine SystemDokument56 SeitenChapter 8 The Endocrine SystemSainuddinSaddinNoch keine Bewertungen
- Return To The Medical Biochemistry PageDokument8 SeitenReturn To The Medical Biochemistry Pagevibhav82Noch keine Bewertungen
- 10 The Adrenal Gland Aldosterone2017 1Dokument27 Seiten10 The Adrenal Gland Aldosterone2017 1Tariq Jamil KoraiNoch keine Bewertungen
- Adrenal Gland: Dr. Fatimah Eliana, SPPD, KemdDokument46 SeitenAdrenal Gland: Dr. Fatimah Eliana, SPPD, KemdZahra AstriantaniNoch keine Bewertungen
- 1 - Biokimia ReproduksiDokument56 Seiten1 - Biokimia ReproduksiTarissa Kartika EdwinNoch keine Bewertungen
- Endokrinologi: Zulkhah NoorDokument121 SeitenEndokrinologi: Zulkhah NoorShafiraNoch keine Bewertungen
- Adrnl StressDokument43 SeitenAdrnl StressPraveen ChowdharyNoch keine Bewertungen
- Anatomy and Physiology of The Adrenal GlandsDokument18 SeitenAnatomy and Physiology of The Adrenal GlandsAnastazia AdeelaNoch keine Bewertungen
- Chapter 25 - Introduction To MetabolismDokument5 SeitenChapter 25 - Introduction To Metabolismtomorrow.today.yesterday .yesterdayNoch keine Bewertungen
- Endocrine Pathophysiology Nursing NotesDokument4 SeitenEndocrine Pathophysiology Nursing Notesgrad_nurse_2015100% (2)
- Mineralocorticoids: Shajeer. SDokument42 SeitenMineralocorticoids: Shajeer. SShajeer SalimNoch keine Bewertungen
- Animal Histology - EndocrineDokument17 SeitenAnimal Histology - EndocrineDanielle FletcherNoch keine Bewertungen
- Homeostasis 121117100359 Phpapp02Dokument68 SeitenHomeostasis 121117100359 Phpapp02midahrazalNoch keine Bewertungen
- Endocrinologi Part 2 2015Dokument74 SeitenEndocrinologi Part 2 2015Annisa Tria FadillaNoch keine Bewertungen
- Hormone 1. OverviewDokument3 SeitenHormone 1. OverviewLê Phạm Uyển NhãNoch keine Bewertungen
- Adrenal Gland: From Wikipedia, The Free EncyclopediaDokument8 SeitenAdrenal Gland: From Wikipedia, The Free Encyclopediadujana rastanawiNoch keine Bewertungen
- Biology: a QuickStudy Laminated Reference GuideVon EverandBiology: a QuickStudy Laminated Reference GuideBewertung: 3 von 5 Sternen3/5 (2)
- Endocrinology SlidesDokument344 SeitenEndocrinology SlidesleanNoch keine Bewertungen
- Chronic Renal Failure TutorialDokument85 SeitenChronic Renal Failure TutorialFrances Rose Cabrera SalongaNoch keine Bewertungen
- Endo 3 Notes PDFDokument9 SeitenEndo 3 Notes PDFDilNoch keine Bewertungen
- Adrenal HormonesDokument62 SeitenAdrenal HormonesM.PRASAD NAIDUNoch keine Bewertungen
- HT Age Based PDFDokument8 SeitenHT Age Based PDFDoni TrinandaNoch keine Bewertungen
- Zalameda - Hypothalamic and Pituitary Agents Adrenocortical Agents Thyroid and Parathyroid Anti Diabetic AgentsDokument55 SeitenZalameda - Hypothalamic and Pituitary Agents Adrenocortical Agents Thyroid and Parathyroid Anti Diabetic AgentsNicole ObispoNoch keine Bewertungen
- National Standard Examination in Junior Science (Nsejs) Ijso Stage-IDokument11 SeitenNational Standard Examination in Junior Science (Nsejs) Ijso Stage-IRashad AlamNoch keine Bewertungen
- Untitled16 PDFDokument17 SeitenUntitled16 PDFElizabeth LeonNoch keine Bewertungen
- Chapter 27: Fluid, Electrolyte, and Acid-Base HomeostasisDokument37 SeitenChapter 27: Fluid, Electrolyte, and Acid-Base HomeostasisAli AftabNoch keine Bewertungen
- Solved Second Sessional Question Paper of Pharmacology-II Theory (BP503T)Dokument12 SeitenSolved Second Sessional Question Paper of Pharmacology-II Theory (BP503T)ANUJ KUMARNoch keine Bewertungen
- Signal Molecule TrackerDokument4 SeitenSignal Molecule Trackerarman_azad_2Noch keine Bewertungen
- Lab 6td5tbyyvtc1920 RevDokument15 SeitenLab 6td5tbyyvtc1920 RevrinidinanNoch keine Bewertungen
- ENDOCRINE SYSTEM - Anatomy and PhysiologyDokument10 SeitenENDOCRINE SYSTEM - Anatomy and PhysiologyJay Crishnan Morales CajandingNoch keine Bewertungen
- Lect.8 HyperaldosteronismDokument10 SeitenLect.8 HyperaldosteronismShubhamNoch keine Bewertungen
- And Its: Dr. V.PushkarDokument92 SeitenAnd Its: Dr. V.PushkarMudit MisraNoch keine Bewertungen
- Renal Tubular AcidosisDokument6 SeitenRenal Tubular AcidosisRichella Khansa Lauditta100% (1)
- PNLE: Maternal and Child Health Nursing Exam 2Dokument6 SeitenPNLE: Maternal and Child Health Nursing Exam 2Darnell Adrian EstobioNoch keine Bewertungen
- Assessment and Management of Patients With Endocrine DisordersDokument91 SeitenAssessment and Management of Patients With Endocrine DisordersAirme Raz AlejandroNoch keine Bewertungen
- Chapter 27: Fluid and Electrolyte BalanceDokument26 SeitenChapter 27: Fluid and Electrolyte BalanceMarwan M.Noch keine Bewertungen
- 2020-Biochem-Activity-16 - BIOCHEMISTRY OF HORMONESDokument32 Seiten2020-Biochem-Activity-16 - BIOCHEMISTRY OF HORMONESGabrielle John HernaezNoch keine Bewertungen
- Essentials of Anatomy and Physiology 6th Edition Scanlon Test BankDokument15 SeitenEssentials of Anatomy and Physiology 6th Edition Scanlon Test Bankamitydairyzy100% (29)
- Adrenal GlandDokument2 SeitenAdrenal GlandAurelia AlexandraNoch keine Bewertungen
- Adrenal Hormone 2018 PDFDokument66 SeitenAdrenal Hormone 2018 PDFrosyidafiaNoch keine Bewertungen
- English Sistem EndocrineDokument9 SeitenEnglish Sistem EndocrineNurlaili YaniNoch keine Bewertungen
- Losartan Plus HydrochlorothiazideDokument18 SeitenLosartan Plus Hydrochlorothiazidegmsanto7Noch keine Bewertungen
- PNLE: Maternal and Child Health Nursing Exam 1: Text ModeDokument47 SeitenPNLE: Maternal and Child Health Nursing Exam 1: Text ModeAngelica SorianoNoch keine Bewertungen
- P3 Biologi Trial SB PPDokument12 SeitenP3 Biologi Trial SB PPFerguson TehNoch keine Bewertungen
- Renin-Angiotensin System: I: (The Juxtaglomerular Apparatus)Dokument1 SeiteRenin-Angiotensin System: I: (The Juxtaglomerular Apparatus)reioctabianoNoch keine Bewertungen
- Acid-Base and Potassium HomeostasisDokument8 SeitenAcid-Base and Potassium HomeostasisVeronica WongNoch keine Bewertungen
- Introduction To ElectrolytesDokument13 SeitenIntroduction To ElectrolytesMaam ShaNoch keine Bewertungen