Beruflich Dokumente
Kultur Dokumente
AS 05 Qu-1
Hochgeladen von
William LinOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
AS 05 Qu-1
Hochgeladen von
William LinCopyright:
Verfügbare Formate
QUESTIONSHEETS
CHEMISTRY
AS Level
ENERGETICS I
AS TOPIC 5
Questionsheet
ENTHALPY OF DISPLACEMENT
16 marks
Questionsheet
THERMOMETRIC TITRATION
15 marks
Questionsheet
ENTHALPY OF FORMATION
12 marks
Questionsheet
ENTHALPY OF COMBUSTION
12 marks
Questionsheet
BOND DISSOCIATION ENTHALPY
16 marks
Questionsheet
HESSS LAW AND ENTHALPY DIAGRAMS
20 marks
Questionsheet
HESSS LAW CALCULATIONS
15 marks
Questionsheet
HESSS LAW WITH CALORIMETRY
18 marks
Questionsheet
HESSS LAW WITH BOND DISSOCIATION ENTHALPY
15 marks
Questionsheet
10
TEST QUESTION I
19 marks
Questionsheet
11
TEST QUESTION II
19 marks
Authors
Trevor Birt
Donald E Caddy
Andrew Jones
Curriculum Press Licence Agreement:
Paper copies of the A-Level Chemistry Questionsheets
may be copied free of charge by teaching staff or students
for use within their school, provided the Photocopy
Masters have been purchased by their school. No part of
these Questionsheets may be reproduced or transmitted,
in any other form or by any other means, without the
prior permission of the publisher. All rights are reserved.
This license agreement is covered by the laws of England
and Wales Curriculum Press March 2008.
John Brockington
Kevin Frobisher
Andy Shepherd
Stuart Barker
Adrian Bond
Editors
John Brockington
Stuart Barker
CP ress
urriculum
www.curriculum-press.co.uk
Curriculum Press Bank House 105 King Street Wellington Shropshire TF1 1NU
TOPIC 5 Questionsheet 1
AS Level
Do not
write in
margin
ENTHALPY OF DISPLACEMENT
An experiment was carried out to find the enthalpy change for the reaction:
Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s)
The temperature of 20.0 cm3 of copper sulfate solution (1.0 mol dm-3) in a plastic cup was measured every
minute for 3 minutes. Exactly on the third minute 1.95g of zinc powder was added rapidly and the mixture stirred.
The temperature every half-minute was noted for a further 3 minutes.
a) Calculate:
(i) the mol copper ion used
....................................................................................................................................................................... [1]
(ii) the mol zinc used
....................................................................................................................................................................... [1]
b) Calculate and explain the mol used in the calculation
...........................................................................................................................................................................
....................................................................................................................................................................... [2]
Temperature (oC)
c) The following graph was drawn :
Time (min)
(i) Why was the zinc added after 3 minutes?
....................................................................................................................................................................... [1]
(ii) Explain how the value of the temperature change ?T was derived from the graph
....................................................................................................................................................................... [1]
(iii) Explain why the method in (ii) was necessary
....................................................................................................................................................................... [1]
TOTAL (Continued..)
/ 16
AS Level
TOPIC
TOPIC
5 Questionsheet
5 Questionsheet
1 Continued
Do not
write in
margin
ENTHALPY OF DISPLACEMENT
d) The value of T was derived as 39.5 0C.
(i)
Calculate the joules of energy exchange with the surroundings (assuming that 1 cm3 solution requires
4.2 J to increase the temperature by 1 0C).
...........................................................................................................................................................................
...........................................................................................................................................................................
....................................................................................................................................................................... [2]
(ii) Calculate a value for the enthalpy of reaction
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
....................................................................................................................................................................... [4]
e) If the experiment was repeated using magnesium instead of zinc, how and why would you expect the enthalpy
of reaction to compare with your value in d)(ii) ?
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
....................................................................................................................................................................... [3]
TOTAL / 16
AS Level
TOPIC 5 Questionsheet 2
THERMOMETRIC TITRATION
Do not
write in
margin
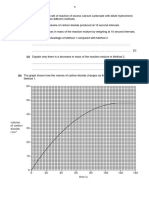
a) The following results were obtained from an experiment to determine the heat of neutralisation of an acid
with an alkali:
Hydrochloric acid added to 50 cm3 of 2.0 M sodium hydroxide
Volume of acid added / cm3
0.0
5.0
10.0
15.0
20.0
25.0
30.0
35.0
40.0
45.0
50.0
(i)
Temperature of mixture / 0C
17.6
22.0
26.0
30.0
33.4
35.4
34.0
33.0
31.6
30.8
29.6
Plot a graph of temperature against volume of acid added and use the graph to determine the volume of
acid required to neutralise the 50 cm3 of 2.0 M sodium hydroxide.
Volume of acid required = .........................................................................................................................[5]
TOTAL (Continued..)
/ 15
AS Level
TOPIC
TOPIC
5 Questionsheet
5 Questionsheet
2 Continued
Do not
write in
margin
THERMOMETRIC TITRATION
(ii) Calculate the molar concentration of the acid.
...........................................................................................................................................................................
...........................................................................................................................................................................
....................................................................................................................................................................... [3]
(iii) Calculate the enthalpy of neutralisation of the acid by the alkali. (Take the specific heat capacity of
dilute solutions to be 4.18 J g-1 K-1)
...........................................................................................................................................................................
...........................................................................................................................................................................
....................................................................................................................................................................... [3]
b) To confirm this result, 40 cm3 of 2 M hydrochloric acid were put into a Thermos flask and its temperature
recorded.Then 60 cm3 of 2 M sodium hydroxide were added and the temperature rose by 10.4 0C. The flask
was rinsed out and 100 cm3 of water put in together with a small electrical heating coil. The coil supplied 30
Joules every second and after 145 seconds the same temperature rise was achieved. Use the electrical data to
calculate the enthalpy of neutralisation of the acid by the alkali.
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
....................................................................................................................................................................... [4]
TOTAL / 15
AS Level
TOPIC 5 Questionsheet 3
Do not
write in
margin
ENTHALPY OF FORMATION
a) Define standard enthalpy of formation of a compound.
...........................................................................................................................................................................
...........................................................................................................................................................................
....................................................................................................................................................................... [3]
b) Calculate the standard enthalpy change for the oxidation of ammonia according to the equation:
4NH3(g) + 5O2(g) 4NO(g) + 6H2O(g)
Standard enthalpies of formation are as follows:
NO(g) = +90 kJ mol-1
H2O(g) = -242 kJ mol-1
NH3(g) = -46 kJ mol-1
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
....................................................................................................................................................................... [3]
c) Ethyne burns in oxygen according to the equation:
2C2H2(g) + 5O2(g) 4CO2(g) + 2H2O(l)
Calculate the standard enthalpy of combustion of this gas given the following standard enthalpies of formation:
CO2(g) = -394 kJ mol-1
H2O(l) = -286 kJ mol-1
C2H2(g) = + 228 kJ mol-1
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
....................................................................................................................................................................... [3]
d) Calculate the standard enthalpy of formation of magnesium carbonate, using the following information.
The standard enthalpy of combustion of magnesium is 602 kJ mol-1 and that of carbon is 394 kJ mol-1.
The standard enthalpy change for the decomposition of magnesium carbonate in the reaction:
MgCO3(s) MgO(s) + CO2(g)
is +100 kJ mol-1.
...........................................................................................................................................................................
...........................................................................................................................................................................
....................................................................................................................................................................... [3]
TOTAL / 12
AS Level
TOPIC 5 Questionsheet 4
Do not
write in
margin
ENTHALPY OF COMBUSTION
a) Define standard enthalpy of combustion of an element or compound.
...........................................................................................................................................................................
...........................................................................................................................................................................
....................................................................................................................................................................... [3]
b) Given the following data:
2C(s) + O2(g) 2CO(g); H = - 222 kJ mol-1
2CO(g) + O2(g) 2CO2(g); H = - 566 kJ mol-1
what is the standard enthalpy of combustion of carbon?
...........................................................................................................................................................................
....................................................................................................................................................................... [2]
c) The standard enthalpy changes for the combustion of decane, octane and ethene are 6778, -5470 and 1411
kJ mol-1 respectively. Use these data to calculate the standard enthalpy change for the cracking of decane:
C10H22(l) C8H18(l) + C2H4(g)
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
....................................................................................................................................................................... [3]
d) In the presence of a nickel catalyst, but-1-ene can be hydrogenated to give butane:
CH2=CHCH2CH3(g) + H2(g) CH3CH2CH2CH3(g)
(i)
Calculate the molar enthalpy of hydrogenation of but-1-ene, given the following standard enthalpies of
combustion:
Hc /kJ mol-1
C4H8(g)
- 2718
H2(g)
- 286
- 2877
C4H10(g)
...........................................................................................................................................................................
...........................................................................................................................................................................
....................................................................................................................................................................... [2]
(ii) State, giving a reason, how you would expect the enthalpy of hydrogenation to change if platinum were
to be used as a catalyst instead of nickel.
Expected change .................................................................................................................................... [1]
Reason .......................................................................................................................................................
............................................................................................................................................................... [1]
TOTAL / 12
AS Level
TOPIC 5 Questionsheet 5
Do not
write in
margin
BOND DISSOCIATION ENTHALPY
a) Define the bond dissociation enthalpy of the HH bond.
...........................................................................................................................................................................
....................................................................................................................................................................... [2]
b) Distinguish between the bond dissociation enthalpy of the OH bond in water and the mean bond dissociation
enthalpy of the OH bond in water.
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...................................................................................................................................................................... [3]
c) Arrange the following in increasing order of mean bond dissociation enthalpy:
(i) CC, C=C, CC
....................................................................................................................................................................... [1]
(ii) CCl, CBr, CI
....................................................................................................................................................................... [1]
d) What is the relationship between bond dissociation enthalpy and (i) bond strength, and (ii) bond length?
Bond strength................................................................................................................................................. [1]
Bond length ................................................................................................................................................... [1]
e) The combustion of propanone is given by the equation:
CH3
CH3
C=O (g) + 4O2(g) 3CO2(g) + 3H2O(g)
Use the following mean bond dissociation enthalpies (all in kJ mol-1) to calculate the enthalpy change for this
reaction:
CC 346 CH 414 C=O 804 O=O 498 OH 463
[7]
TOTAL / 16
AS Level
TOPIC 5 Questionsheet 6
Do not
write in
margin
HESSS LAW & ENTHALPY DIAGRAMS
a) State Hesss law.
...........................................................................................................................................................................
...........................................................................................................................................................................
....................................................................................................................................................................... [4]
b) (i)
Hesss law is in accordance with the first law of thermodynamics. State this law.
...........................................................................................................................................................................
....................................................................................................................................................................... [2]
(ii) Explain why, if Hesss law were not obeyed, the first law of thermodynamics would be violated.
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
....................................................................................................................................................................... [3]
c) (i)
Write an equation, with state symbols, for the combustion of propane.
....................................................................................................................................................................... [2]
(ii) Sketch an enthalpy diagram for the formation and combustion of propane. (Hint The formation of propane
is exothermic.) Clearly label start and finish on your diagram, and show two routes from start to
finish.
[6]
(iii) Apply Hesss law to your diagram in c) (ii) and hence calculate the standard enthalpy of formation of
propane, given the following standard enthalpies of combustion (all in kJ mol-1):
C(s) = -394
H2(g) = -286 C3H8(g) = -2222
...........................................................................................................................................................................
...........................................................................................................................................................................
....................................................................................................................................................................... [3]
TOTAL / 20
AS Level
TOPIC 5 Questionsheet 7
Do not
write in
margin
HESSS LAW CALCULATIONS
In this question you will need the following data.
Standard enthalpy of formation /kJ mol-1
H2O(l)
286
H2S(g)
20.2
Standard enthalpy of combustion/kJ mol-1
S (rhombic)
296.9
S (monoclinic)
297.2
Molar enthalpy of vaporisation of water = 41.1 kJ mol-1
a) (i)
There are two solid allotropes (i.e. crystalline forms) of sulfur, described as rhombic sulfurand monoclinic
sulfur. Construct an enthalpy diagram or cycle, and apply Hesss law to it so as to calculate the standard
enthalpy change for the conversion of rhombic sulfur to monoclinic sulfur.
[4]
(ii) From your answer to a) (i) state, with reason, which allotrope of sulfur is the more stable under standard
conditions.
More stable form ................................................................................................................................... [1]
Reason .................................................................................................................................................. [1]
TOTAL(Continued....)
/ 15
TOPIC 5 Questionsheet 7 Continued
Do not
write in
margin
HESSS LAW CALCULATIONS
b) Monoclinic sulfur is formed in volcanic regions by reaction between sulfur dioxide and hydrogen sulfide according to the equation:
SO2(g) + 2H2S(g) 2H2O(g) + 3S(monoclinic).
Draw an enthalpy diagram or cycle and calculate the standard enthalpy change for this reaction.
[5]
c) For the oxidation of sulfur dioxide to sulfur trioxide according to the equation:
2SO2(g) + O2(g) 2SO3(g); Hreaction = -196.2 kJ mol-1.
From an enthalpy diagram or cycle calculate the standard enthalpy of formation of sulfur trioxide from monoclinic sulfur.
[4]
TOTAL / 15
AS Level
TOPIC 5 Questionsheet 8
Do not
write in
margin
HESSS LAW WITH CALORIMETRY
A student poured exactly 50 cm3 of 2 M hydrochloric acid into a polystyrene cup and recorded its temperature
each minute for three minutes. At 3 minutes, 0.24 g of magnesium turnings was added. The mixture was stirred
and further temperature readings taken until a total time of nine minutes had elapsed. The recorded temperatures
were as follows.
Time / minutes
Temperature / 0C
20
19.7 19.5 19.2
4
43.8
43.0 42.3
41.5 40.8 40.0
a) Plot a graph of temperature against time using these results and hence determine the maximum temperature
change accompanying the reaction.
Maximum temperature change ..............................................................................................................[5]
TOTAL (Continued...)
/ 16
AS Level
TOPICTOPIC
5 Questionsheet
5 Questionsheet
8 Continued
Do not
write in
margin
HESSS LAW WITH CALORIMETRY
b) Use the value from a) and the data given in the question to calculate the enthalpy change for the reaction.
(Assume the density of all solutions to be 1 g cm-3 and their specific heat capacity to be 4.2 J g-1 K-1)
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
....................................................................................................................................................................... [3]
c) In a similar experiment, 0.01 mol of magnesium carbonate was added to exactly 50 cm3 of 2 M hydrochloric
acid in a polystyrene cup. The maximum temperature rise was 5.2 0C. Calculate the enthalpy change for the
reaction.
...........................................................................................................................................................................
...........................................................................................................................................................................
....................................................................................................................................................................... [2]
d) Write a balanced chemical equation to represent the standard enthalpy of formation for magnesium carbonate.
....................................................................................................................................................................... [2]
e) Given the additional data for the standard enthalpies of formation of H2O(l) and CO2(g) as 286 and 394 kJ
mol-1 respectively, together with the reactions above, construct an enthalpy cycle or diagram to show how the
standard enthalpy of formation for magnesium carbonate can be calculated.
[4]
f) Apply Hesss law to the cycle or diagram you have devised and the results from b) and c) to calculate the
standard enthalpy of formation for magnesium carbonate.
...........................................................................................................................................................................
...........................................................................................................................................................................
....................................................................................................................................................................... [2]
TOTAL / 18
AS Level
TOPIC 5 Questionsheet 9
HESSS LAW WITH BOND DISSOCIATION ENTHALPY
Do not
write in
margin
Ethanol production in the UK is mainly from the hydration of ethene. In this process, ethene and steam are
reacted together at 300 0C and 70 atm pressure in the presence of phosphoric acid, which acts as a catalyst:
CH2=CH2(g) + H2O(g) C2H5OH(l)
a) Apply Hesss law to an enthalpy cycle or diagram to calculate the enthalpy change for this reaction, given the
following data:
Standard enthalpy of formation of ethanol = -278 kJ mol-1
C(s) C(g); H = + 715 kJ mol-1
Mean bond dissociation enthalpies / kJ mol-1:
HH = 436 O
O = 496 C
C = 612 OH = 464 CH = 413
[8]
b) Calculate the enthalpy change for the same reaction, using the following standard enthalpies of combustion:
CH2=CH2 = 1411 kJ mol-1
CH3CH2OH = 1368 kJ mol-1
...........................................................................................................................................................................
...........................................................................................................................................................................
....................................................................................................................................................................... [3]
c) Why is the value calculated from bond dissociation enthalpies in a) different from that obtained in b)?
...........................................................................................................................................................................
....................................................................................................................................................................... [1]
d) For the formation of gaseous ethanol, CH2=CH2(g) + H2O(g) C2H5OH(g)
would you expect the enthalpy of reaction to be more negative or less negative than the value for the formation
of liquid ethanol? Give your reason.
More or less negative .................................................................................................................................... [1]
Reason ...............................................................................................................................................................
....................................................................................................................................................................... [2]
TOTAL / 15
AS Level
TOPIC 5 Questionsheet 10
Do not
write in
margin
TEST QUESTION I
NOTE In this Questionsheet, assume that dilute aqueous solutions have a specific heat capacity of 4.18 J g-1 K-1
and a density of 1.00 g cm-3.
a) (i)
Define the term molar enthalpy of solution.
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
....................................................................................................................................................................... [3]
(ii) When 5.35 g of ammonium chloride was dissolved in 100 g of water, the temperature fell from 20.55 0C
to 17.10 0C. Calculate the molar enthalpy of solution of ammonium chloride.
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
....................................................................................................................................................................... [3]
(iii) What is the significance of the sign of the value calculated in (ii)?
...........................................................................................................................................................................
....................................................................................................................................................................... [1]
b) 50.0 cm3 of 0.5 mol dm-3 NH3(aq) at 20.00 oC. was mixed with 50.0 cm3 0.5 mol dm-3 HCl(aq), also at 20.00 oC.
The temperature of the mixture rose to 23.12 oC. Calculate the molar enthalpy of neutralisation of HCl(aq) by
NH3(aq).
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
....................................................................................................................................................................... [3]
TOTAL (Continued..)
/ 17
AS Level
TOPIC 5 Questionsheet 10 Continued
Do not
write in
margin
TEST QUESTION I
c) (i)
Write an equation to represent the formation of 1 mole of solid ammonium chloride from its elements in
their standard states.
....................................................................................................................................................................... [1]
(ii) Use the following data, together with your answers from a) and b), to calculate the molar enthalpy of
solution of ammonia. Your answer should include an enthalpy cycle or diagram to which Hesss law is
clearly applied.
Hf / kJ mol-1 : NH3(g) = -43.2
HCl(g) = -92.3
NH4Cl(s) = -315
Molar enthalpy of solution of HCl(g) = -187 kJ mol-1
Enthalpy cycle or diagram
[5]
Application of Hesss law
............................................................................................................................................................... [1]
Molar enthalpy of solution of ammonia
...................................................................................................................................................................
............................................................................................................................................................... [2]
TOTAL / 19
AS Level
TOPIC 5 Questionsheet 11
Do not
write in
margin
TEST QUESTION II
A spirit burner containing ethanol was weighed. 80 cm3 of water was measured into a metal calorimeter and the
temperature measured. The burner was ignited under the calorimeter and extinguished after 5 minutes.
The temperature of the water and the mass of the burner were then immediately measured. The following results
were obtained:
Mass of spirit burner at start (g)
Mass of spirit burner after combustion (g)
Temperature of water at start (oC)
o
Temperature of water after combustion ( C)
32.44
31.96
16.4
33.1
a) Calculate
(i) the mass of ethanol burned
...........................................................................................................................................................................
....................................................................................................................................................................... [1]
(ii) the temperature rise of the water
...........................................................................................................................................................................
....................................................................................................................................................................... [1]
TOTAL(Continued...)
/ 17
AS Level
TOPIC 5 Questionsheet 11 Continued
Do not
write in
margin
TEST QUESTION II
b) Assuming 1cm3 of water rises 1oC for 4.2 J energy , calculate
(i) the energy exchange with the surroundings (in joules)
...........................................................................................................................................................................
....................................................................................................................................................................... [2]
(ii) the energy yield for ethanol in joules per g
...........................................................................................................................................................................
....................................................................................................................................................................... [2]
(iii) the enthalpy of combustion for ethanol
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
....................................................................................................................................................................... [4]
(iv) Explain why it is not possible to state that the answer to (iii) is thestandard enthalpy of combustion.
...........................................................................................................................................................................
....................................................................................................................................................................... [2]
(v) Give an assumption used when calculating an answer to (i)
....................................................................................................................................................................... [1]
c) If the standard enthalpy of combustion is -1400 kJ mol-1, suggest and explain two reasons for the difference.
Reason 1 ....................................................................................................................................................... [1]
Explanation ......................................................................................................................................................
....................................................................................................................................................................... [2]
Reason 2 ....................................................................................................................................................... [1]
Explanation ......................................................................................................................................................
....................................................................................................................................................................... [2]
TOTAL / 19
Das könnte Ihnen auch gefallen
- 0620 w11 QP 61Dokument16 Seiten0620 w11 QP 61Andrew HarrisonNoch keine Bewertungen
- 0620 w12 QP 63Dokument12 Seiten0620 w12 QP 63nicole1003Noch keine Bewertungen
- University of Cambridge International Examinations International General Certifi Cate of Secondary EducationDokument12 SeitenUniversity of Cambridge International Examinations International General Certifi Cate of Secondary EducationSumaira AliNoch keine Bewertungen
- Q2 Chem Lecture 4Dokument4 SeitenQ2 Chem Lecture 4Laiba ImtiazNoch keine Bewertungen
- University of Cambridge International Examinations General Certificate of Education Ordinary LevelDokument20 SeitenUniversity of Cambridge International Examinations General Certificate of Education Ordinary Levelmstudy123456Noch keine Bewertungen
- 4335 03 PDFDokument16 Seiten4335 03 PDFshahidperwezNoch keine Bewertungen
- Chem G-9 Lesson 7 IGCSE Qs - Rates of ReactionDokument24 SeitenChem G-9 Lesson 7 IGCSE Qs - Rates of ReactionKarim WaelNoch keine Bewertungen
- June 1999 Paper 4Dokument16 SeitenJune 1999 Paper 4YasirNoch keine Bewertungen
- 9701 s14 QP 21 PDFDokument8 Seiten9701 s14 QP 21 PDFNor AfidahNoch keine Bewertungen
- t2 Chem Revision Ex 19Dokument16 Seitent2 Chem Revision Ex 19Nicholas OwNoch keine Bewertungen
- Projek Skor Kimia 2014 Siri 8 eDokument13 SeitenProjek Skor Kimia 2014 Siri 8 eZul BaidiNoch keine Bewertungen
- University of Cambridge International Examinations International General Certifi Cate of Secondary EducationDokument12 SeitenUniversity of Cambridge International Examinations International General Certifi Cate of Secondary EducationHaider AliNoch keine Bewertungen
- Titration 2Dokument3 SeitenTitration 2Mushfiqur RahmanNoch keine Bewertungen
- Energetics Exam QsDokument5 SeitenEnergetics Exam Qszadinova.tereza16Noch keine Bewertungen
- Using Conc. of Solutions in Mol (DM) - 3 QPDokument15 SeitenUsing Conc. of Solutions in Mol (DM) - 3 QPw276Noch keine Bewertungen
- 2 1 A Student Investigates Two Fuels: Hexane and Octane.: 5070/42/M/J/18 © UCLES 2018Dokument14 Seiten2 1 A Student Investigates Two Fuels: Hexane and Octane.: 5070/42/M/J/18 © UCLES 2018Mujeeb SiddiqueNoch keine Bewertungen
- Chemistry: University of Cambridge International Examinations General Certificate of Education Ordinary LevelDokument16 SeitenChemistry: University of Cambridge International Examinations General Certificate of Education Ordinary Levelmstudy123456Noch keine Bewertungen
- 5070 s09 QP 4Dokument16 Seiten5070 s09 QP 4mstudy1234560% (1)
- Time Temperature Superposition TTS MethoDokument42 SeitenTime Temperature Superposition TTS MethoErick CamachoNoch keine Bewertungen
- 5070 s14 QP 42Dokument16 Seiten5070 s14 QP 42Fatima AliNoch keine Bewertungen
- 5070 w15 QP 42Dokument16 Seiten5070 w15 QP 42Basith FarhanNoch keine Bewertungen
- مشروع مرجينDokument58 Seitenمشروع مرجينpk9zg2rxkgNoch keine Bewertungen
- Unit 4 (Mole) PAPER 4Dokument118 SeitenUnit 4 (Mole) PAPER 4Muhammad Hasnain SikandarNoch keine Bewertungen
- University of Cambridge International Examinations General Certifi Cate of Education Advanced Subsidiary Level and Advanced LevelDokument12 SeitenUniversity of Cambridge International Examinations General Certifi Cate of Education Advanced Subsidiary Level and Advanced LevelHubbak KhanNoch keine Bewertungen
- My TestDokument33 SeitenMy TestqusaielnoorNoch keine Bewertungen
- Ocr 30672 PP 08 Jun L Gce 2816 01Dokument12 SeitenOcr 30672 PP 08 Jun L Gce 2816 01Philip_830Noch keine Bewertungen
- 0620 s15 QP 63Dokument12 Seiten0620 s15 QP 63Nada HadiNoch keine Bewertungen
- 0620 w12 QP 61Dokument16 Seiten0620 w12 QP 61n0tsewNoch keine Bewertungen
- Rate of Reaction 2 QP (Tomek)Dokument9 SeitenRate of Reaction 2 QP (Tomek)Tomasz OstrowskiNoch keine Bewertungen
- Cambridge International Advanced Subsidiary and Advanced LevelDokument16 SeitenCambridge International Advanced Subsidiary and Advanced LevelCallie Jia LiNoch keine Bewertungen
- 9701 m16 QP 52Dokument8 Seiten9701 m16 QP 52SanthiKalyanaGrantNoch keine Bewertungen
- 9701 s06 QP 2Dokument14 Seiten9701 s06 QP 2G M Ali KawsarNoch keine Bewertungen
- Read These Instructions First: 7 Printed PagesDokument7 SeitenRead These Instructions First: 7 Printed PagesZainab ShigriNoch keine Bewertungen
- IGCSE Chem Summer 2016 Question Paper 63Dokument12 SeitenIGCSE Chem Summer 2016 Question Paper 63rNoch keine Bewertungen
- Revision Heat of Neutralisation 123Dokument6 SeitenRevision Heat of Neutralisation 123Ariyan ShahmieNoch keine Bewertungen
- Cambridge International AS & A Level: CHEMISTRY 9701/51Dokument16 SeitenCambridge International AS & A Level: CHEMISTRY 9701/51Suraj sssNoch keine Bewertungen
- Chemistry Paper 2 TZ1 SL May2014Dokument24 SeitenChemistry Paper 2 TZ1 SL May2014Liam Antonio VazquezNoch keine Bewertungen
- Chemistry: University of Cambridge International Examinations General Certificate of Education Ordinary LevelDokument16 SeitenChemistry: University of Cambridge International Examinations General Certificate of Education Ordinary Levelmstudy123456Noch keine Bewertungen
- c2 Chapter 4 HigherDokument20 Seitenc2 Chapter 4 HigherChitrashiNoch keine Bewertungen
- Bab 07 - k2 - Struktur Dan EseiDokument30 SeitenBab 07 - k2 - Struktur Dan EseiazharsarahNoch keine Bewertungen
- CC2014Dokument12 SeitenCC2014syron.nandoo.eduNoch keine Bewertungen
- SPM Chemistry QuestionDokument6 SeitenSPM Chemistry QuestionSaya MenangNoch keine Bewertungen
- Chemistry Paper 3 TZ1 SLDokument28 SeitenChemistry Paper 3 TZ1 SLMotiani VanshikaNoch keine Bewertungen
- Bgcse ChemistryDokument320 SeitenBgcse Chemistrybaone segaetshoNoch keine Bewertungen
- Cambridge International Advanced Subsidiary and Advanced LevelDokument8 SeitenCambridge International Advanced Subsidiary and Advanced Leveljesse koduaNoch keine Bewertungen
- 5124 w07 QP 3Dokument16 Seiten5124 w07 QP 3Sudibyo GunawanNoch keine Bewertungen
- 0620 s02 QP 6Dokument12 Seiten0620 s02 QP 6Amanda0375Noch keine Bewertungen
- CAPE Chemistry Study Paper 001αDokument36 SeitenCAPE Chemistry Study Paper 001αJerome JAcksonNoch keine Bewertungen
- 5070 s04 QP 4Dokument16 Seiten5070 s04 QP 4mstudy123456Noch keine Bewertungen
- 1kadar Tindak BalasDokument20 Seiten1kadar Tindak BalasJulaiha Abdul HamidNoch keine Bewertungen
- Rate of Reaction 3 QPDokument9 SeitenRate of Reaction 3 QPPirate HunterNoch keine Bewertungen
- Separate Chemistry: Higher Tier in BoldDokument16 SeitenSeparate Chemistry: Higher Tier in BoldzipperNoch keine Bewertungen
- Pahang STPM Trial 2011 Chemistry Paper 2 (W Ans)Dokument21 SeitenPahang STPM Trial 2011 Chemistry Paper 2 (W Ans)lawrenceNoch keine Bewertungen
- 0620 w10 QP 63Dokument12 Seiten0620 w10 QP 63Haider AliNoch keine Bewertungen
- Ii Term QP 9Dokument20 SeitenIi Term QP 9G M Ali KawsarNoch keine Bewertungen
- Metal Catalysed Carbon-Carbon Bond-Forming ReactionsVon EverandMetal Catalysed Carbon-Carbon Bond-Forming ReactionsNoch keine Bewertungen
- Potential of Carabao Grass (Paspalum Conjugatum) As Bioethanol FeedstockDokument61 SeitenPotential of Carabao Grass (Paspalum Conjugatum) As Bioethanol FeedstockmayheartNoch keine Bewertungen
- AERO188 Questions 7Dokument3 SeitenAERO188 Questions 7fitri suciNoch keine Bewertungen
- Lactic Acid TR 2015Dokument27 SeitenLactic Acid TR 2015Huỳnh Thị Thu HiềnNoch keine Bewertungen
- Evaluation of Anti-Inflammatory Activity of Canthium Parviflorum by In-Vitro MethodDokument3 SeitenEvaluation of Anti-Inflammatory Activity of Canthium Parviflorum by In-Vitro MethodYanie IsfahannyNoch keine Bewertungen
- Chapter Fourteen: Fundamentals of General, Organic, and Biological Chemistry 5th EditionDokument37 SeitenChapter Fourteen: Fundamentals of General, Organic, and Biological Chemistry 5th EditionSCReddyNoch keine Bewertungen
- ChERD PaperDokument15 SeitenChERD PaperSuleman AhmadNoch keine Bewertungen
- Water Vapor Permeability, Tensile Properties and Solubility of Methylcellulose-Based Edible FilmsDokument8 SeitenWater Vapor Permeability, Tensile Properties and Solubility of Methylcellulose-Based Edible FilmsMaulana Karnawidjaja WahyuNoch keine Bewertungen
- Chembio Activity 2Dokument2 SeitenChembio Activity 2Adrian VillanuevaNoch keine Bewertungen
- Bannari - Dis - Eia - Eng StartupDokument12 SeitenBannari - Dis - Eia - Eng StartupSajeevNoch keine Bewertungen
- Organic PDFDokument15 SeitenOrganic PDFAmira AbdallahNoch keine Bewertungen
- Chemistry EsterizationDokument4 SeitenChemistry EsterizationStrindy GlasgowNoch keine Bewertungen
- Chapter2 CarboncompoundsDokument71 SeitenChapter2 CarboncompoundsJachinta JuliusNoch keine Bewertungen
- IntroductionDokument16 SeitenIntroductionAkhtar RazaNoch keine Bewertungen
- Activity C05: Evaporation and Intermolecular Attractions (Temperature Sensor)Dokument9 SeitenActivity C05: Evaporation and Intermolecular Attractions (Temperature Sensor)JellyShapesNoch keine Bewertungen
- Sterilization of Ortho Instruments / Orthodontic Courses by Indian Dental AcademyDokument57 SeitenSterilization of Ortho Instruments / Orthodontic Courses by Indian Dental Academyindian dental academyNoch keine Bewertungen
- Nathan Lisbin Synthetic FFR #2 Synthesis of A Coumarin Laser DyeDokument10 SeitenNathan Lisbin Synthetic FFR #2 Synthesis of A Coumarin Laser DyeNate LisbinNoch keine Bewertungen
- Paper Chromatography ExperimentDokument5 SeitenPaper Chromatography Experimentsiguro hwahahahNoch keine Bewertungen
- OXIDATIONDokument36 SeitenOXIDATIONMansab AliNoch keine Bewertungen
- Is 17342Dokument10 SeitenIs 17342AnuradhaPatraNoch keine Bewertungen
- Delhi Excise Act, 2009Dokument31 SeitenDelhi Excise Act, 2009manoj1035Noch keine Bewertungen
- OSPAR List of Substances / Preparations Used and Discharged Offshore Which Are Considered To Pose Little or No Risk To The Environment (PLONOR)Dokument5 SeitenOSPAR List of Substances / Preparations Used and Discharged Offshore Which Are Considered To Pose Little or No Risk To The Environment (PLONOR)MaychelBetaWNoch keine Bewertungen
- CB 504course MaterialDokument270 SeitenCB 504course MaterialAnonymous u3sNldnR100% (1)
- Preparatory Problems Icho 2013Dokument177 SeitenPreparatory Problems Icho 2013Kang PentolNoch keine Bewertungen
- Gel SerumDokument6 SeitenGel SerumLydya UtariNoch keine Bewertungen
- FMDS-07-31 (02.2020) - Storage of Aerosol ProductsDokument60 SeitenFMDS-07-31 (02.2020) - Storage of Aerosol ProductsFogo NeptúnNoch keine Bewertungen
- Acerit Mamauag Sibal Soriano PED.Dokument111 SeitenAcerit Mamauag Sibal Soriano PED.Justine DaquioagNoch keine Bewertungen
- OSD-OPM-Manual REVISED 10/2015Dokument23 SeitenOSD-OPM-Manual REVISED 10/2015Jesse BeardNoch keine Bewertungen
- 11Dokument7 Seiten11Shyam TannaNoch keine Bewertungen
- Success Point Science Academy: Chemistry Time: 1.00 HR Marks: 100Dokument4 SeitenSuccess Point Science Academy: Chemistry Time: 1.00 HR Marks: 100Brahmanand TiwariNoch keine Bewertungen
- 3VQ S4CLD2005 BPD en UsDokument43 Seiten3VQ S4CLD2005 BPD en UsAnonymousNoch keine Bewertungen