Beruflich Dokumente
Kultur Dokumente
Thermoplastic Processing of Proteins
Hochgeladen von
Arlette PinedaOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Thermoplastic Processing of Proteins
Hochgeladen von
Arlette PinedaCopyright:
Verfügbare Formate
R: Concise Reviews and in Food Science
JFS R: Concise Reviews/Hypotheses in Food Science
Thermoplastic Processing of Proteins for Film FormationA Review
V.M. HERNANDEZ-IZQUIERDO AND J.M. KROCHTA
ABSTRACT: Increasing interest in high-quality food products with increased shelf life and reduced environmental impact has encouraged the study and development of edible and/or biodegradable polymer films and coatings. Edible films provide the opportunity to effectively control mass transfer among different components in a food or between the food and its surrounding environment. The diversity of proteins that results from an almost limitless number of side-chain amino-acid sequential arrangements allows for a wide range of interactions and chemical reactions to take place as proteins denature and cross-link during heat processing. Proteins such as wheat gluten, corn zein, soy protein, myofibrillar proteins, and whey proteins have been successfully formed into films using thermoplastic processes such as compression molding and extrusion. Thermoplastic processing can result in a highly efficient manufacturing method with commercial potential for large-scale production of edible films due to the low moisture levels, high temperatures, and short times used. Addition of water, glycerol, sorbitol, sucrose, and other plasticizers allows the proteins to undergo the glass transition and facilitates deformation and processability without thermal degradation. Target film variables, important in predicting biopackage performance under various conditions, include mechanical, thermal, barrier, and microstructural properties. Comparisons of film properties should be made with care since results depend on parameters such as film-forming materials, film formulation, fabrication method, operating conditions, testing equipment, and testing conditions. Film applications include their use as wraps, pouches, bags, casings, and sachets to protect foods, reduce waste, and improve package recyclability. Keywords: compression molding, edible film, extrusion, protein, thermoplastic processing
Introduction
n edible film can be defined as a thin layer of edible material, which can be formed on a food as a coating or preformed as a film that can be placed between food components, used as a food wrap, or formed into a pouch to contain foods. Its purpose is to inhibit migration of moisture, oxygen, carbon dioxide, aromas, and lipids; carry food ingredients (for example, antioxidants, antimicrobials, and flavors); and/or improve mechanical integrity or handling characteristics of the food (Krochta and De Mulder-Johnston 1997). Materials used to produce edible films can be divided into 4 categories: biopolymer hydrocolloids, lipids, resins, and composites. Biopolymer hydrocolloids include proteins such as gelatin, keratin, collagen, casein, soy protein, whey protein, myofibrillar proteins, wheat gluten, and corn zein; and polysaccharides such as starch, starch derivatives, cellulose derivatives, and plant gums. Suitable lipids include waxes, acylglycerols, and fatty acids. Resins include shellac and wood rosin. Composites generally contain both lipid and hydrocolloid components in the form of a bilayer or an emulsion (Krochta and others 1994; Perez-Gago and Krochta 2005). Formation of edible films can be achieved by 2 main processes. The wet process involves biopolymer dispersion or solubilization in a film-forming solution (solution-casting) followed by evaporation of the solvent. The dry process relies on the thermoplastic behavior that some proteins and polysaccharides display at low mois-
ture levels in compression molding and extrusion (Cuq and others 1997b; Pommet and others 2005; Liu and others 2006). The objective of this article is to review the literature available on compression molding and extrusion of vegetable proteins (corn zein, wheat gluten, soy protein) and animal proteins (milk proteins, myofibrillar proteins, collagen, gelatin), and to identify the critical material- and process-related factors and physicochemical mechanisms that affect the film-making process. Properties and applications of the resulting film structures are also reviewed.
Material-Related Factors
Proteins
Proteins exist in all living cells. They are a major constituent in skin, nerve tissue, tendons, muscle, hair, blood, enzymes, and hormones (Stevens 1999). They are also present in plants to provide structure and biological activity (Krochta 2002). Therefore, proteins are a renewable, biodegradable/edible resource with great potential to improve the quality and stability of a large range of food products by using a number of processing techniques. For a long time, proteins have been used to produce edible materials, but understanding of the precise physical and chemical mechanisms of protein interactions continues to evolve (Ar as 1992; Li and Lee 1996; e Redl and others 1999a; Pommet and others 2005). Proteins are heteropolymers, with -amino acids being their monomer units. With the 20 amino acids most commonly found in proteins (see Table 1), an almost limitless number of sequential arrangements with a wide range of interactions and chemical reactions is possible (McMurry 1994; Stevens 1999; Pommet and others 2003). In this regard, proteins differ from polysaccharides, where only a few monomers are involved in the range of polysaccharides
C 2008 Institute of Food Technologists doi: 10.1111/j.1750-3841.2007.00636.x
MS 20070640 Submitted 8/20/2007, Accepted 11/3/2007. Authors are with Dept. of Biological and Agricultural Engineering, Univ. of California, Davis, Davis, CA 95616, U.S.A. Author Krochta is also with Dept. of Food Science and Technology, Univ. of California, Davis, Davis, CA 95616, U.S.A. Direct inquiries to author Krochta (E-mail: jmkrochta@ucdavis.edu).
R30
JOURNAL OF FOOD SCIENCEVol. 73, Nr. 2, 2008
Further reproduction without permission is prohibited
Protein processing for lm formation . . .
that exist. The most common polysaccharides, cellulose and starch, contain only 1 monomer, glucose. During heat processing, proteins disaggregate, denature, dissociate, unravel, and align in the direction of the flow. These changes allow the protein molecules to recombine and cross-link through specific linkages (Ar as 1992; Redl e and others 1999a). The cross-linking reactions can result in a high glass transition temperature and high melt viscosity, which require addition of plasticizers to increase free volume and mobility of the molecules. As temperature increases above the glass transition, the plasticized proteins turn into a soft, rubbery material that can be shaped into desired forms. Upon cooling, the matrix network gets fixed into the desired structure (Pommet and others 2003). Corn zein. Zein is the prolamin protein fraction found in corn gluten. It has a molecular weight of 18 to 45 kDa and is soluble in 60% to 70% ethanol. The poor solubility of zein in water is due to its high content of hydrophobic amino acid residues leucine, alanine, and proline (Kokini and others 1994). Wheat gluten. Wheat gluten is composed of the water-insoluble prolamin and glutelin protein fractions known as gliadin and glutenin, respectively. The molecular weight of gliadin is in the range of 20 to 50 kDa, while glutenin has an average molecular weight of 250 kDa. The high molecular weight in glutenin can be attributed to the presence of intermolecular disulfide bonds, joining individual protein chains and resulting in a larger polymer (Kokini and others 1994; Krochta 1997). Soy protein. The most common way to classify soy proteins is based on their sedimentation rate in fractional ultracentrifugation. Larger Svedberg (S) numbers indicate a larger protein. Soy protein fractions include 2S, 7S, 11S, and 15S. The main constituents of soy protein are the 7S (conglycinin) and 11S (glycinin) fractions. -conglycinin has a molecular weight of 180 kDa and is rich in asparagine, glutamine, leucine, and arginine residues. Unlike glycinin, which contains 20 intramolecular disulfide bonds, disulfide cross-linking in conglycinin is limited (Krochta 1997; Khorshid and others 2007). Milk proteins. Milk proteins can be divided into casein and whey protein. Casein represents 80% of the total milk protein and consists of , , and -casein with molecular weights between 19 and 25 kDa. The low cysteine levels in casein result in little disulfide
Table 1 --- Common amino acids found in proteins. Name Neutral Alanine Asparagine Cysteine Glutamine Glycine Isoleucine Leucine Methionine Phenylalanine Proline Serine Threonine Tryptophan Tyrosine Valine Basic Arginine Histidine Lysine Acidic Aspartic acid Glutamic acid Abbreviations Ala (A) Asn (N) Cis (C) Gln (Q) Gly (G) Ile (I) Leu (L) Met (M) Phe (F) Pro (P) Ser (S) Thr (T) Try (W) Tyr (Y) Val (V) Arg (R) His (H) Lys (K) Asp (D) Glu (E) Molecular weight 89 132 121 146 75 131 131 149 165 115 105 119 204 181 117 174 155 146 133 147
cross-linking and an open random-coil structure. The high proline content results in better emulsifying properties compared to whey protein (Khwaldia and others 2004). Whey protein comprises 20% of the milk protein and is the protein that remains soluble after casein has been precipitated at pH 4.6. Whey protein includes -lactoglobulin (M w = 18 kDa), -lactalbumin (M w = 14 kDa), bovine serum albumin (M w = 66 kDa), immunoglobulins, and proteose-peptones. Whey proteins are globular and heat labile in nature (Krochta 1997). Myofibrillar proteins. Fish has been the main source of myofibrillar proteins for edible film formation. Regardless of the source (mammalian or fish), the main proteins found in the muscle are myosin (500 kDa) and actin. These proteins are obtained after removing other components such as blood, lipids, myoglobin, and collagen through a series of washing treatments. Fibrous proteins (myosin, F-actin) can form films with good mechanical properties, while globular proteins such as G-actin need to be unfolded first (Lacroix and Cooksey 2005). Collagen and gelatin. Collagen is a fibrous protein found in hides, tendon, cartilage, bone, and connective tissue. Two major components consisting of 2 types of covalent cross-linked chain pairs have been identified: (M w = 100 kDa) and (M w = 200 kDa) (Lacroix and Cooksey 2005). A unique characteristic in collagen is the occurrence of glycine in every 3rd amino acid residue throughout most of its structure (Krochta 1997). Hydrolysis of collagen produces gelatin with molecular weights from 3 to 200 kDa depending on the raw material used and the handling conditions (Lacroix and Cooksey 2005).
Plasticizers
Plasticizers are generally added to the protein matrix to improve processability and to modify the properties of the final structure. As opposed to internal plasticizers, which are copolymerized or reacted with the polymer, external plasticizers consist of low molecular weight, low volatility substances that interact with the polymer chains producing swelling (Sothornvit and Krochta 2005). Water is the most effective plasticizer in biopolymer materials, enabling them to undergo the glass transition, facilitating deformation, and processability of the biopolymer matrix. Without water addition, the temperature region of thermal degradation would be easily reached before films could be formed (Tolstoguzov 1993). However, an excessive amount of water during protein extrusion would decrease melt viscosity, which, in turn, would lead to low motor torque and specific mechanical energy input, resulting in low product temperature that could reduce the degree of protein transformation and interactions. Besides water, common plasticizers for edible films include monosaccharides, oligosaccharides, polyols, lipids, and derivatives (Sothornvit and Krochta 2005). Plasticizer composition, size, shape, and ability to attract water have been shown to affect solution-cast whey protein film barrier properties (Sothornvit and Krochta 2000). Glycerol (C 3 H 8 O 3 ) is a low molecular weight, hydrophilic plasticizer that has been widely used in the thermoplastic processing of proteins (Redl and others 1999a; Cunningham and others 2000; Zhang and others 2001; Pommet and others 2003, 2005; Sothornvit and others 2003, 2007; Hernandez-Izquierdo 2007). Its high plasticizing effect has been attributed to the ease with which glycerol can insert and position itself within the 3-dimensional biopolymer network (di Gioia and Guilbert 1999). Sucrose (C 12 H 22 O 11 ) and sorbitol (C 6 H 14 O 6 ) have also been studied for their plasticizing effects, including plasticizing fish myofibrillar proteins to produce biopackaging materials by thermal compression-molding (Cuq and others 1997b). Pommet and others (2005) tested several compounds with
Vol. 73, Nr. 2, 2008JOURNAL OF FOOD SCIENCE R31
Adapted from Stevens (1999), p 101314.
R: Concise Reviews and in Food Science
R: Concise Reviews and in Food Science
Protein processing for lm formation . . .
different chemical functions, number of functional groups, and degree of hydrophobicity as wheat gluten plasticizers (Table 2). From this study, the critical factors for a good plasticizer were found to be low melting point, low volatility, and protein compatibility. In addition to these characteristics, permanence in the film and amount of plasticizer needed should be taken into account when choosing a good plasticizer (di Gioia and Guilbert 1999; Sothornvit and Krochta 2001). The efficiency with which a plasticizer affects specific mechanical and barrier properties can be quantified (Sothornvit and Krochta 2001). The relative effect on mechanical and barrier properties can vary a great amount among different plasticizers. Table 2 summarizes the plasticizers that have been used in the thermoplastic processing of proteins. were flexible and partially soluble, while films produced at 140 C were also flexible but nearly insoluble. A higher solubility and hydration would be desirable in the edible film packaging of dry soup mixes or controlled drug delivery. Insoluble films could be used as wraps or casings for foods with somewhat higher moisture content. Pommet and others (2005) prepared wheat gluten blends with 5 different plasticizers (water, glycerol, 1,4-butanediol, octanoic acid, and lactic acid) in a mixing chamber at a speed of 100 rpm and temperatures of 80 and 60 C for hydrated and dry gluten, respectively. The blends were mixed for 5 min after reaching maximum torque. After mixing, the blends were compression-molded at 100 C for 5 min or 130 C for 15 min. While mixing induced both aggregation (due to temperatures > 60 C) and deaggregation of gluten molecules (due to shear), the thermal compression under static conditions resulted only in gluten aggregation, as indicated by an increase in the sodium dodecyl sulfate (SDS)-insoluble gluten fraction. Corn gluten meal, which is rich in proteins, particularly zein, was blended with polar plasticizers (water, glycerol) and amphiphilic plasticizers (octanoic and palmitic acids, dibutyl tartrate and phthalate, and diacetyl tartaric acid ester of monodiglycerides [DATEM]) at 25 rpm for 6 min. The mixing chamber wall was controlled at 80 C with circulating water. Compression molding was then used to produce bars (60 5 2 mm) at 110 to 130 C, 1.4 MPa, and a dwell time of 10 min. Different degrees of plasticizer exudation were detected depending on the plasticizer type (di Gioia and Guilbert 1999). Rakotonirainy and Padua (2001) used compression molding to obtain 1- to 4-ply laminated zein sheets. The individual film components were obtained by solution-casting while the pressing was carried out in a Carver Press at 120 C for 5 min. The lamination process induced melting and flow of the oleic acidzein films, decreasing voids and defects. As a result, mechanical and oxygen permeability properties were improved. Pol and others (2002) took advantage of the thermoplastic properties and differences in molecular weight of soy protein and corn zein to produce single- and double-coat laminates by compression molding. The higher-molecular-weight soy protein was plasticized with glycerol, thermally compacted at a temperature of 150 C and
Process-Related Factors
Compression molding
Processing conditions dictate the type and extent of physical and chemical modifications that take place during thermoplastic processing of proteins (Moraru and Kokini 2003). The combination of high temperatures, high pressures, short times, and low moisture contents in compression molding causes the transformation of proteinplasticizer mixtures into viscoelastic melts. The protein films are then formed upon cooling through hydrogen, ionic, hydrophobic, and covalent interactions (Pol and others 2002). Compression-molded soy protein isolateglycerol films were produced at an optimum temperature of 150 C, a pressure of 10 MPa, and a dwell time of 2 min (Cunningham and others 2000). Thermogravimetric analysis (TGA) indicated that soy protein degraded at temperatures above 180 C (Ogale and others 2000). Similar conditions were used to form compression-molded whey protein isolateglycerol films, but dwell times above 2 min resulted in film degradation at high temperatures. Above 140 C, film degradation occurred at both the minimum (0.81 MPa) and the maximum (2.25 MPa) pressures studied (Sothornvit and others 2003). However, no films could be formed at 104 C with dwell times below 2 min. The use of higher compression molding temperatures promotes a more extensive protein denaturation resulting in higher cross-linking and reduced solubility. Sothornvit and others (2003) found that whey protein isolateglycerol films obtained at 104 C
Table 2 --- Plasticizers used in the thermoplastic processing of proteins. Plasticizer 1,4-Butanediol DATEMa Dibutyl tartrate Dibutyl phthalate Glycerol Protein studied Wheat gluten Corn gluten meal (rich in zein) Corn gluten meal (rich in zein) Corn gluten meal (rich in zein) Corn gluten meal (rich in zein) Wheat gluten Soy protein Soy protein Wheat gluten Wheat gluten Wheat gluten Whey protein Whey protein Wheat gluten Corn gluten meal (rich in zein) Wheat gluten Corn gluten meal (rich in zein) Myobrillar proteins Myobrillar proteins Corn gluten meal (rich in zein) Wheat gluten Processing method Compression molding Compression molding Compression molding Compression molding Compression molding Extrusion Compression molding Extrusion Extrusion Compression molding Compression molding Compression molding Extrusion Compression molding Compression molding Compression molding Compression molding Compression molding Compression molding Compression molding Compression molding Reference Pommet and others (2005) di Gioia and Guilbert (1999) di Gioia and Guilbert (1999) di Gioia and Guilbert (1999) di Gioia and Guilbert (1999) Redl and others (1999a) Cunningham and others (2000) Zhang and others (2001) Pommet and others (2003) Pommet and others (2003) Pommet and others (2005) Sothornvit and others (2003) Hernandez-Izquierdo (2007) Pommet and others (2005) di Gioia and Guilbert (1999) Pommet and others (2005) di Gioia and Guilbert (1999) Cuq and others (1997b) Cuq and others (1997b) di Gioia and Guilbert (1999) Pommet and others (2005)
Lactic acid Octanoic acid Palmitic acid Sorbitol Sucrose Water
a
DATEM, diacetyl tartaric acid ester of monodiglycerides.
R32
JOURNAL OF FOOD SCIENCEVol. 73, Nr. 2, 2008
Protein processing for lm formation . . .
used as the base film. The zein lamination was carried out at 125 C without significant degradation of the soy protein film. Compression molding can result in the formation of proteinbased films with a range of mechanical and barrier properties that are dependent on the formulation and processing conditions used. It is a suitable technology to investigate the thermoplastic properties of plasticized proteins as well as the properties of the resulting films. Compression molding can also serve as a step toward the use of a more continuous, high-speed technology for film manufacture such as extrusion. A comparison between single- and twin-screw extrusion of oleic acid-zein (70 g oleic acid/100 g zein) films showed that the higher pressure and shearing force developed inside the twin-screw extruder barrel resulted in a higher tensile strength (about 4 MPa), a more compact, smoother, and more uniform structure, while samples formed by single-screw extrusion presented a large number of pinholes as well as lower tensile strength (3 MPa) (Wang and Padua 2002). The thermoplastic behavior of wheat gluten plasticized with glycerol has been investigated under mixing, compressionmolding, and corotating self-wiping twin-screw extrusion conditions (Redl and others 1999a, 1999b; Pommet and others 2003). Redl and others (1999a) examined the influence of feed rate (1.9, 4.9, and 8.1 kg/h), screw speed (50, 100, and 200 rpm), and barrel temperature (40, 60, and 80 C) on die pressure, product temperature, residence time, and specific mechanical energy. Gluten powder was fed with a volumetric feeder, while glycerol was injected using a peristaltic pump at a constant level of 53 1.2%. Changes in feed rates were limited by the initial conveyance of the gluten powder in the feeding zone. For instance, at a feed rate of 4.9 kg/h and a screw speed of 50 rpm, the extruder feeding hopper filled up and resulted in extrusion instabilities. High screw speeds led to remarkable viscous heat dissipation, which increased the product temperature beyond desirable levels, resulting in extrudate rupture. Depending on operating conditions, rod-like extrudates ranged from very smoothedsurfaced extrudates with high swell to completely disrupted extrudates (Redl and others 1999a). Pommet and others (2003) related the disrupted appearance of extrudates obtained under similar conditions as those described by Redl and others (1999a) to the cross-linking of gluten proteins that resulted from high product temperature and significant mechanical energy input. The specific mechanical energy input (SME), which is the energy provided by the motor drive to the material in the extruder per unit mass (SME = net torque screw speed/mass flow rate, kJ/kg), constitutes an important parameter for comparing different combinations of extrusion conditions. The SME input will have a direct impact in the rheological properties of the melt, the extent of macromolecular transformations, and interactions among components, resulting in extrudates that could range from partially soluble to insoluble, expanded, or even degraded structures. Pommet and others (2003) found that the degree of induced cross-linking in wheat gluten proteins was affected by specific mechanical energy and product temperature during extrusion of wheat gluten plasticized with glycerol. To distinguish the influence of temperature and mechanical energy on protein cross-linking kinetics, they studied the activation energies in compression molding, which involves temperature and pressure, compared to mixing, which involves temperature and mechanical energy. The activation energy for the mixing process (33.7 kJ/mol) was much lower than that obtained for compression molding (170 kJ/mol). This suggests that mechanical energy plays an important role in favoring interactions among proteins, increasing their reactivity, and, therefore, lowering the cross-linking activation energy. A multistep process to form soy protein isolate sheets involved thorough mixing of soy protein isolate, water, glycerol, and other additives, followed by compounding twin-screw extrusion. This process resulted in pellets that were finally extruded into sheets using a single-screw extruder operated at 20 to 25 rpm. The barrel temperature varied from 120 to 160 C, while the die temperature was 100 to 120 C, depending on the formulation (Zhang and others 2001). A patent on edible soy protein-based casings proposed extruding the film-forming materials through a circular opening to
Vol. 73, Nr. 2, 2008JOURNAL OF FOOD SCIENCE R33
Extrusion
Extrusion is one of the most important polymer processing techniques in use today. Most synthetic plastics, such as LDPE (low density polyethylene) films, are processed in extruders and generally pass through two or more extruders before the finished product is obtained (Robertson 1993). Therefore, extrusion of edible/biodegradable films would increase their commercial potential, offering several advantages over solution-casting. An extruder constitutes a specialized form of continuous HTST (hightemperature short-time) reactor (Fichtali and van de Voort 1989) where the raw materials are continuously introduced into a hopper, conveyed by a screw, and pushed through a die of a desired shape. The process can involve any or all of the following operations: heating, cooling, feeding, conveying, compressing, shearing, reacting, mixing, melting, homogenizing, amorphousizing (converting polymer crystalline domains to amorphous domains), cooking, and shaping. The extruder barrel can be subdivided into 3 processing zones: (1) the feeding zone, where the granular, lowdensity raw material is introduced into the barrel and slightly compressed with expelling of air; (2) the kneading zone, with further compression, a higher degree of fill, and increasing pressure, temperature, and material-density; and finally, (3) the heating zone, where the highest shear rates, temperatures, and pressures are achieved along with the final product texture, color, density, and functional properties (Hauck and Huber 1989). Controllable or process variables include screw speed, screw configuration (location and dimensions of individual screw elements such as conveying elements and kneading blocks along the shaft), screw length-todiameter ratio (L/D), barrel temperature profile, moisture addition, feed rates, and die size/shape. Dependent, response, or system parameters include product temperature, residence time distribution, torque, specific mechanical energy input (SME), pressure at the die, and degree of screw fill. Finally, target variables can be subdivided into product characteristics (for example, moisture content, color, texture, flavor, nutritional value) and economic response variables (Fichtali and van de Voort 1989). For film formation, target variables include moisture content, mechanical, barrier, and color properties, among others. Dry, granular zein resins containing a mixture of fatty acids (39.9% linoleic acid, 15.6% oleic acid, 13.6% palmitic acid, 5.4% linolenic acid, and 5.2% stearic acid) were extruded into sheets using a corotating twin-screw extruder with 5 heating zones. Barrel temperatures in zones 1, 2, and 3 were kept constant at 40, 80, and 120 C, respectively. Processing variables under study included barrel temperatures in zones 4 and 5 (110, 120, and 130 C), amount of solvent (70% aqueous ethanol) added (20%, 30%, and 40% [v/w]), and screw speed (150, 200, and 250 rpm). Increasing extrusion temperature increased the tensile strength but decreased the elongation of the extruded sheets. Although added solvent decreased melt viscosity and improved processability during extrusion, there were no significant effects on tensile properties. Screw speed was also not significant in this study (Ha and Padua 2001).
R: Concise Reviews and in Food Science
R: Concise Reviews and in Food Science
Protein processing for lm formation . . .
obtain a tubular extrudate that could be blown and stretched to obtain a film (Naga and others 1996). Soy protein mixtures have also been formulated to obtain solid (as opposed to flexible films) edible/biodegradable articles such as cups, trays, plates, spoons, knives, and forks by thermoplastic processes such as extrusion and compression molding (Jane and Wang 1996; Jane and Zhang 1998). Whey protein isolate sheets plasticized with glycerol and water were obtained using a corotating twin-screw extruder and a slit die. Melt temperature at the time of sheet formation was between 143 and 150 C. Sheets obtained under different glycerol contents (46 to 52% glycerol dwb) and screw speeds (200 to 275 rpm) were flexible, transparent, and displayed thermoplastic behavior that allowed them to be compression-molded into thinner films. Such films could then be heat-sealed with an impulse heat-sealer (Hernandez-Izquierdo 2007). This type of sealing is generally required for stand-alone films that require some cooling time before the sealing bars are released. Heat-sealable whey protein-based films could be formed into pouches to contain preweighed ingredients such as milk-based powders or other nondairy dry mixes. The latter would require proper labeling to warn consumers who might be allergic to these types of proteins. Although single- and twin-screw extruders have been used successfully in the fabrication of protein-based films, twin-screw extruders offer improved conveying and mixing capabilities and interchangable screw profiles created by slipping individual screw sections onto parallel shafts. These characteristics often result in films with improved mechanical, barrier, and microstructural properties. The next section describes in more detail the physicochemical mechanisms (structural transformations, phase transitions, and interactions among components) that take place during thermoplastic processing of proteins. 4. The coiled spring theory, which explains plasticizing effects from the point of view of tangled macromolecules. At low moisture contents, water can act as an antiplasticizer by increasing the structural order of polymer systems. Kristo and others (2007) observed an increase in elastic modulus (E) and tensile strength (TS) of pullulansodium caseinate films in the moisture content range of 5% to 8%. These small amounts of water filled holes created in the biopolymer matrix under large deformation. At higher contents, the presence of water in the polymer network produced a plasticizing effect. Antiplasticization effects can also be observed at very low temperatures. Zhang and others (2001) found an increase in the storage modulus (E ) of extruded soy protein sheets due to crystallization of the waterglycerol mixture, resulting in stiffening of the sheets.
Protein interaction
The various possible ways in which proteins may interact during thermoplastic processing are unclear. The reactivity of proteins depends on their physicochemical environment as well as the thermomechanical treatment. Plasticizer effect. The type and amount of plasticizer used can have an effect on protein reactivity as well as extrudate properties. A study on the influence of plasticizer type on the reactivity (aggregation/deaggregation) of gluten proteins showed that octanoic (at levels of 25%, 30%, and 35% weight/total weight) and lactic (at levels of 20%, 30%, and 35% weight/total weight) acids inhibited protein aggregation in spite of the high mixing temperatures used, which otherwise promote protein aggregation. Further thermal treatment (compression molding) resulted in higher aggregation of gluten proteins plasticized with octanoic acid than gluten proteins plasticized with lactic acid. The pH of gluten plasticized with octanoic acid was 4.4 compared to 2.4 with lactic acid. The aggregation of gluten proteins is thought to involve sulfhydryl/disulfide interchange reactions. Since S works as a catalyst, such reactions are not favored by acidic conditions (Pommet and others 2005). Sothornvit and others (2003) reported that compression-molded whey protein films plasticized with water (at levels of 30%, 40%, and 50% of total mixture weight) resulted in brittle and insoluble films, while films plasticized with glycerol (at levels of 30%, 40%, and 50% of total mixture weight) were flexible and their solubility depended on the temperatures used to form the films. Extrusion of whey protein-based sheets with 46%, 49%, and 52% glycerol in dry basis was successfully achieved by injecting a liquid mixture consisting of 70% glycerol and 30% water into the 2nd section of a twin-screw extruder barrel. The 1st section of the extruder was used to feed whey protein isolate powder that was mixed later on with the liquid mixture at different feed rates depending on the formulation under study. Temperatures were gradually increased along the barrel. Water contents in the liquid mixture higher than 30% resulted in product expansion, while feeding 100% glycerol affected processability, producing high torque and discoloration of the films (Hernandez-Izquierdo 2007). Ha and Padua (2001) extruded corn zein and fatty acids (1:0.75, w/w) into sheets. Stable extrusion runs could be achieved due to fatty acid binding onto the zein surface, as suggested by differential scanning calorimetry (DSC). The binding phenomenon prevented lipid phase separation and sticking of the zein onto the extruder barrel. Temperature and moisture effects. Proteins are also sensitive to changes in temperature, which can degrade proteins, and moisture, which acts as a plasticizer. High temperatures and low moisture contents can result in protein degradation during
nlike polysaccharides, where the only reactive group is the hydroxyl group, proteins offer a large range of possible interactions and chemical reactions. Proteins can participate in chemical reactions through covalent (peptide and disulfide) linkages and non-covalent interactions through ionic, hydrogen, and van der Waals bonding. In addition, hydrophobic interactions occur between nonpolar groups of amino acid chains (Kokini and others 1994).
Physicochemical Mechanisms
Plasticization
To reduce the brittleness and improve processability of proteinbased materials, addition of plasticizers is generally required (Pommet and others 2005). The plasticizing effect of small polar molecules such as glycerol and water has been described in terms of insertion and positioning within the 3-dimensional protein network. The following plasticizing mechanisms have been proposed to describe the effect of plasticizers on the protein network (di Gioia and Guilbert 1999; Sothornvit and Krochta 2005): 1. The lubricity theory, where the plasticizer is seen as acting as a lubricant to facilitate mobility of the chain molecules past one another. 2. The gel theory, which considers the disruption of polymer polymer interactions (hydrogen bonds and van der Waals or ionic forces). 3. The free volume theory, which considers that the plasticizer increases the free volume and mobility of polymer chains. This theory has been used to understand the effect of plasticizers in lowering the glass transition temperature.
R34 JOURNAL OF FOOD SCIENCEVol. 73, Nr. 2, 2008
Protein processing for lm formation . . .
thermoplastic processing. Kokini and others (1994) studied the glass transitions of zein, gliadin, and glutenin as a function of moisture content and developed state diagrams that can be used to predict protein behavior during processes such as extrusion. At the beginning of an extrusion process, the protein is fed in a dry, granular, solid-like state. As the protein enters the extruder and comes in contact with plasticizer and moisture at increasing temperatures, the polymer molecules gain mobility and undergo a glass-to-rubber transition. The result is corresponding changes in rheological properties, more specifically, the display of viscoelastic behavior. Increasing the temperature and/or moisture content can further allow the proteinplasticizerwater mixture to pass from the rubbery state into the free-flow region. As the material emerges from the die, elastic response becomes the governing factor again. From a molecular point of view, the transformation from a glassy to a rubbery state corresponds to an increase in disorder, free volume, and mobility of the polymer molecules. These modifications in molecular organization result in variations of the material physical properties such as thermal, mechanical, and dielectrical properties (Cuq and others 1997a). DSC to characterize protein interactions. A technique widely used to characterize the thermal transitions of a polymer is differential scanning calorimetry (DSC). Protein and proteinplasticizer thermal transitions detected by DSC include the glass transition temperature (Tg), melting, crystallization, thermal denaturation, aggregation, and protein degradation. DSC thermograms of extruded soy protein sheets evidenced the plasticizing effect of water as higher moisture contents decreased the Tg of the sheets. DSC also suggested complete denaturation of the soy protein during extrusion processing as a single Tg was detected instead of the 2 characteristic Tg values for the 7S and the 11S fractions (Zhang and others 2001). Di Gioia and Guilbert (1999) used modulated-DSC to study the zein plasticization by water, glycerol, and octanoic acid plasticizers. Increasing plasticizer concentration decreased Tg. The authors illustrated the typical baseline change at the glass transition for 10%, 20%, and 30% dwb octanoic acid. A study on thermal gelation of whey proteins (Fitzsimons and others 2007) revealed the existence of an exothermic peak that reflects the slow formation of intermolecular bonds (aggregation). This exothermic peak can be easily obscured by the endothermic peak that corresponds to denaturation of the globular proteins. Fitzsimons and others (2007) were able to detect protein aggregation using a Setaram microcalorimeter with a sample size of approximately 850 mg. DSC thermograms showing the effects of different salt concentrations, protein concentrations, and heating rates are presented in this study. DMTA to characterize protein interactions. Predicting the changes in rheological properties that occur as a result of plasticization and processing conditions is essential for process design, evaluation, quality control, and stability of the product. In order to detect and measure the temperatures at which the amorphous domains of proteins reach the free-flow state, methods such as dynamic mechanical thermal analysis (DMTA), based on the fundamentally different responses of viscous and elastic elements at controlled temperature, should be used. DMTA is used to relate structural and viscoelastic properties to changes in temperature, frequency and/or deformation. Moreover, DMTA is capable of detecting glass transitions when, due to the breadth of the transition or the small change in heat capacity, it is not possible to detect such transitions by DSC (Cuq and others 1997a). DMTA was used to study the thermoplastic properties of fish myofibrillar proteins. Significant changes in storage modulus (E ), loss modulus (E ), tan , and sample height were observed between 215 and 250 C and were associated with the glass transition of the dry protein. Similar Tg values have been reported for collagen (200 C, measured by DSC) and gelatin (210 C, measured by DSC), while lower Tg values characterize corn zein (139 C, measured by DMTA), casein (144 C, measured by DMTA), and wheat gluten (160 to 162 C, measured by DMTA). The differences in Tg values can be attributed to structure, molecular weight and organization, and intermolecular interactions in the different proteins (Cuq and others 1997b). Redl and others (1999b) investigated the viscoelastic behavior of wheat gluten plasticized with glycerol above Tg and proposed a network structuring mechanism based on their rheological and biochemical findings. In the 1st stage of mixing, depolymerization and plasticization of proteins occur as a result of the mechanical shear and hydrophilic interactions, which results in gel-like behavior. As temperature and mixing time increase, covalent cross-linking (such as disulfide bonds) leads to an increase in molecular size and the resulting network structure. This structuring mechanism is thought to be temperature controlled, dependent on mechanical energy input but independent of mixing conditions and glycerol content. TGA to characterize protein interactions. Another thermal analysis technique, useful to study the thermal stability of a polymer, is thermogravimetric analysis (TGA). Most commonly, TGA involves continuous weight measurement as the sample temperature increases in air or an inert environment such as nitrogen. At higher temperatures, weight loss can be related to polymer decomposition (Stevens 1999). Ogale and others (2000) used TGA to obtain information on the thermal degradation of soy protein isolate powder and soy protein isolateglycerol films. For pure soy protein isolate powder, the rate of weight loss was small (less than 0.1 wt%/ C) until 180 C, moderate between 180 and 200 C, and significant above 200 C. For plasticized films, the weight loss was small until 150 C, moderate between 150 and 180 C, and significant above 180 C. Based on these findings, the authors established an optimum compression-molding temperature of 150 C for soy protein isolateglycerol mixtures. However, the mixtures themselves were not analyzed, and the processing temperature selected might be affected not only by the pressures used during actual processing but also by the properties of the mixtures as opposed to films. The following section describes the mechanical, thermal, permeability, and microstructural properties of various protein-based films obtained by compression molding and extrusion.
Film Properties
he properties (mechanical, thermal, permeability, and microstructural) of the structures that result from the thermoplastic processing of protein-based materials will depend on the raw materials as well as the processing and testing conditions used.
Mechanical properties
Stress/strain curves that result from film tensile testing provide valuable information on film flexibility, toughness, and elongation, useful in the prediction of the package performance during handling and storage. Mechanical properties of compression-molded soy protein (Cunningham and others 2000; Ogale and others 2000; Pol and others 2002), whey protein (Sothornvit and others 2007), and wheat gluten (Pommet and others 2005) films and extruded soy protein (Zhang and others 2001), corn zein (Wang and Padua 2002), and gelatin/sodium alginate films (Liu and others 2006) have been reported. As glycerol content increased from 20% to 40%, the percent elongation of compression-molded soy protein films increased from 1.5% to 106%, and the tensile strength of the films decreased
Vol. 73, Nr. 2, 2008JOURNAL OF FOOD SCIENCE R35
R: Concise Reviews and in Food Science
R: Concise Reviews and in Food Science
Protein processing for lm formation . . .
from 15.8 to 1.6 MPa. At all plasticizer levels, intensive mixing resulted in higher percent elongation than samples obtained from manually mixed, un-aged mixtures (Cunningham and others 2000). Ogale and others (2000) reported a value of storage modulus (E ) of 5 GPa for 30% glycerolsoy protein isolate compression-molded films at the beginning of the DMA testing (temperature of 100 C). As temperature increased, E , which is related to the stiffness of the film (elastic component), decreased to 0.4 GPa at room temperature (25 C) and 15 MPa at 150 C. Sothornvit and others (2007) found that increasing glycerol content from 40% to 50% in compression-molded whey protein films increased percent elongation from 85% to 94% and decreased tensile strength from 8 to 4 MPa. Molding temperature and pressure did not affect film tensile properties significantly. Zhang and others (2001) also reported a continuous increase in percent elongation with increasing glycerol content in extruded soy protein sheets. As glycerol concentration decreased, tensile strength and elastic modulus increased, resulting in very brittle, difficult-to-process sheets. At 23% glycerol content, the evolution of storage modulus (E ) with temperature was affected by the moisture content of the samples. E of samples with higher moisture contents (13.9% and 26%) decreased greatly as temperature increased. Samples with lower moisture contents (2.8% and 7.7%) showed low temperature dependence in the temperature range of 100 to 130 C. Pommet and others (2005) studied the tensile properties of compression-molded wheat gluten-based sheets plasticized with water, glycerol, 1,4-butanediol, lactic acid, and octanoic acid as a function of the glass transition temperature (Tg) previously determined by DMTA for various plasticizer contents. Higher plasticizer concentrations resulted in lower Tg values and lower tensile strength at break (TS). Depending on the plasticizer type, at a specific concentration, wheat gluten-based sheets were either in the glassy (TS around 30 MPa), rubbery (TS around 5 MPa), or intermediate state. The plasticizer efficiency (extent of Tg decrease upon plasticizer addition) was determined to be lactic acid > butanediol, glycerol, water > octanoic acid. Since wheat gluten sheets plasticized with octanoic acid were in the glassy state, the tensile properties of these sheets were difficult to determine. The storage modulus (E ) seemed to be independent of plasticizer type and content (about 2.5 109 Pa). Tensile strength of twin-screw extruded corn zein films plasticized with oleic acid was higher (4.2 MPa) than that of singlescrew extruded films (3.1 MPa), while percent elongation was lower for twin-screw extruded films (96.3%) when compared to singlescrew extruded films (115.5%). These differences in tensile properties were attributed to the higher pressure and shearing force that can be developed in twin-screw extrusion. Scanning electron microscopy (SEM) images revealed a more compact and uniform structure in samples formed by twin-screw extrusion than samples formed by single-screw extrusion (Wang and Padua 2002). Liu and others (2006) reported that addition of corn oil increased the tensile strength and elastic modulus, while reducing the percent elongation of gelatin/sodium alginate films. These results were explained in terms of a strengthening effect of the oil globules in the protein matrix. Nonetheless, films with 2.5% corn oil had lower tear resistance than films without oil. Tear resistance values reported by Pol and others (2002) include those for soy protein films (19.3 N/cm), polyethylene (289.5 to 772 N/cm), nylon 6 (193 to 347.4 N/cm), cellophane (7.72 to 38.6 N/cm), and cellulose acetate (7.72 to 57.9 N/cm). Addition of either a single- or a double-coat layer of zein did not affect the tear resistance value of the base soy film. Acceptable values for tear resistance will depend on specific applications. Higher tear resistance would be needed for films used to pack sharp products such as frozen foods. Talens and Krochta (2005) compared the plasticizing effects of a viscoelastic wax (beeswax) and an elastic wax (carnauba wax) on the tensile properties of solution-cast whey proteinglycerol films. The waxes were incorporated in the films in the form of an emulsion. Beeswax acted as a plasticizer, since it resulted in lower tensile strength than films without wax. Carnauba wax had an antiplasticizing effect, increasing the elastic modulus of whey proteinglycerol films. Therefore, using beeswax as a plasticizer as well as a water barrier component can result in improved film properties and less use of a hydrophilic plasticizer, such as glycerol. Table 3 summarizes data on tensile properties of various compression-molded and extruded protein films at comparable plasticizer contents and tensile-testing conditions. Properties of selected synthetic films are included for comparison. Although protein films are generally weaker and have lower elongation values than synthetic films, they are adequate to be used as individual wraps or small pouches protected by a secondary package such as a paperboard box.
Table 3 --- Tensile properties of compression-molded and extruded protein lms.a Tensile properties Film formulation 40% Gly-WPIb 50% Gly-WPIb 46% Gly-WPIc 49% Gly-WPIc Oleic acid-zeind 40% Gly-SPIe 50Gly:100 SPIf LDPEg HDPEg Polyesterg PVCg Formation method Compression molding Compression molding Extrusion Extrusion Extrusion Compression molding Extrusion Extrusion ------Tensile strength (MPa) 8 4 4 3.5 4.2 2.6 7.1 13 26 178 93 Elastic modulus (MPa) 144 60 46.5 37 96.4 --144 --------Elongation (%) 85 94 127 121 96.3 74.5 --500 300 85 30
a Abbreviations: Gly = glycerol; WPI = whey protein isolate; SPI = soy protein isolate; LDPE = low density polyethylene; HDPE = high density polyethylene; PVC = polyvinyl chloride. b Sothornvit and others (2007). c Hernandez-Izquierdo (2007). d Wang and Padua (2002). e Cunningham and others (2000). f Zhang and others (2001). g Lacroix and Cooksey (2005).
R36
JOURNAL OF FOOD SCIENCEVol. 73, Nr. 2, 2008
Protein processing for lm formation . . .
Thermal properties
In order to predict protein-based package performance under different end-use conditions ranging from freezing to cooking, the thermal transitions of the films should be investigated. DSC and DMTA have been the most common thermal analysis techniques used in determining the glass transition temperatures of various protein-based films (di Gioia and Guilbert 1999; Ogale and others 2000; Zhang and others 2001). Ogale and others (2000) reported the existence of multiple glass transition temperatures (corresponding to peaks in tan ) in compression-molded soy protein isolate glycerol films. Lower Tg values (around 60 C) were associated with glycerol-dominated phases in local nonhomogeneous regions of the films, while higher Tg values (> 70 C) were associated with the Tg values of soy protein. Attempts to measure Tg by DSC were not successful. Zhang and others (2001) used both DSC and DMTA to study the thermal transitions of extruded soy protein sheets containing different moisture and glycerol levels. At the same glycerol content, DSC indicated that the Tg of the protein sheet decreased with increasing moisture content, revealing the plasticizing effect of water. When moisture content was low, even with added glycerol, the Tg of the soy protein sheets was above room temperature. With 30 parts of glycerol and 2.8% to 26% moisture content, the Tg values of soy protein sheets ranged from 7 to 50 C for the highest and the lowest moisture contents, respectively. During DMTA testing, as temperature increased, moisture was lost from the films, resulting in higher transition temperatures. Therefore, DSC was considered to be a more reliable technique in determining the Tg values of soy protein sheets containing moisture. Hernandez-Izquierdo (2007) investigated the relationship between the thermal transitions and heat-sealability of extruded whey protein-based films. DSC showed the existence of endothermic peaks between 156 and 183 C. Heat-seals were achieved at temperatures between 165 and 204 C. The higher temperatures required to heat-seal the films can be attributed to thickness effects. Table 4 summarizes data on thermal transitions of various compression-molded and extruded protein films. curs during film-formation by compression molding. Compared to glycerol-plasticized solution-cast whey protein films, compressionmolded films were thicker (0.6 to 0.8 mm) and had higher WVP values (around 360 g mm/d kPa m2 ). Soluble protein in glycerolplasticized films was significantly affected by molding temperature, indicating higher cross-linking at higher temperatures. To improve the moisture barrier properties of gelatin/sodium alginate extruded films, Liu and others (2006) incorporated hydrophobic materials (corn oil and olive oil) along with 1% lecithin for emulsification purposes into the waterglycerol mixture used in the formation of films. Only olive oil decreased the water vapor transfer rate (WVTR) in the films. Rakotonirainy and Padua (2001) pressed together 1 to 4 layers of oleic acid-plasticized zein films previously formed by solution casting. Although the fusion-lamination eliminated cracks and pinholes, there was no significant decrease in WVP suggesting that wa, ter vapor molecules migrated through the laminated structure regardless of voids and defects. Lamination of a hydrophilic polymer with a hydrophobic one can result in the decrease of WVP without significant detriment to oxygen barrier properties as shown by Pol and others (2002). The authors conducted a study in which compression-molded soy protein films were either single- or double-coated with corn zein. Compared to the base soy film, WVP values were reduced by 20% for single-coated and by 50% for double coated samples. Table 5 summarizes WVP data of various protein films as well as synthetic films.
Microstructural properties
The microstructural characteristics of protein-based films are a function of the formulations and processing conditions used to manufacture the films. Ogale and others (2000) used atomic force microscopy (AFM) and scanning electron microscopy (SEM) to study the surface texture of compression-molded soy protein films. Surface characteristics ranged from smooth and glassy for pure protein films, rough with scattered air bubbles for 20% glycerol films, homogeneous for 30% glycerol films, to nonhomogeneous for 40% glycerol films. Cross-sections of the films containing 0% and 20% glycerol were smooth, while 30% and 40% glycerol films presented ridges and valleys, which can be related to a more ductile material. Pol and others (2002) studied the microstructure of soy protein isolate/corn zein laminated films. Optical microscopy and field emission scanning electron microscopy findings were in agreement with those reported earlier for unlaminated soy films containing 30% glycerol (Ogale and others 2000). On the other hand, the microstructure of corn zein coatings was smooth and did not present ridges and valleys, indicating a glassy, brittle type of material. SEM images of extruded oleic acidzein sheets showed that single-screw extruded sheets had a large number of pinholes, while
Permeability properties
Compared to edible waxes and most synthetic polymers, protein films have high water vapor permeability (WVP) values. Among different proteins, the lowest WVP corresponds to corn zein, wheat gluten, and fish myofibrillar protein films (Krochta 2002). Sothornvit and others (2003) studied the effect of plasticizer content and processing conditions on WVP and protein solubility of compression-molded whey protein films plasticized with either glycerol or water. WVP was not affected by plasticizer content, molding temperature, or molding pressure. However, films plasticized with glycerol had higher WVP values than those plasticized with water. This effect was attributed to the loss of moisture that oc-
Table 4 --- Thermal transitions of compression-molded and extruded protein lms.a Film formulation 49% Gly-WPIb 40% Gly-SPIc 30Gly:100 SPId
a
Formation method Extrusion Compression molding Extrusion
Thermal analysis technique DSC DMTA DSC
Heating rate ( C/min) 20 2 20
Transition temperatures ( C) 156 to 183 64 71 150 7 (26% moisture) 50 (2.8% moisture)
b Hernandez-Izquierdo (2007). c Ogale and others (2000). d
Abbreviations: Gly = glycerol; WPI = whey protein isolate; SPI = soy protein isolate; DSC = differential scanning calorimetry; DMTA = dynamic mechanical thermal analysis. Zhang and others (2001).
Vol. 73, Nr. 2, 2008JOURNAL OF FOOD SCIENCE
R37
R: Concise Reviews and in Food Science
R: Concise Reviews and in Food Science
Protein processing for lm formation . . .
aging materials would further attract interest by the industry and the consumers. Laminated/coated structures provide the opportunity to exploit the benefits of each of the macromolecular comFormation WVP Film formulation method (g mm/d kPa m2 ) ponents used. Improvements in mechanical and barrier properties can be achieved through the use of a high-molecular-weight, ducWPI:Gly (1:1)b Compression molding 336 WPI:Gly (1.6:1)c Solution-casting 120 tile, hydrophilic protein such as soy or whey protein coated with a WPI:Sor (1.6:1)c Solution-casting 62 lower-molecular-weight, brittle, hydrophobic component such as c Zein:PEG+Gly (2.6:1) Solution-casting 70 to 107 corn zein or a lipid. Fusion of several protein films into 1 structure WG:Gly (2.5:1)c Solution-casting 108 c can provide a stronger, more homogeneous material with fewer deSPI:Gly (1.7:1) Solution-casting 262 fects than a single layer of the same material. Inclusion of nanoparLDPEc Extrusion 0.08 EVOH (68% VOH)c --0.25 ticles or incorporation of polysaccharides can also result in imCellophanec --7.3 proved mechanical properties. Moreover, addition of antimicroa Abbreviations: Gly = glycerol; WPI = whey protein isolate; Sor = sorbitol; bials, antioxidants, and flavors would result in a wider range of food PEG = polyethylene glycol; WG = wheat gluten; SPI = soy protein isolate; applications. LDPE = low density polyethylene; EVOH = ethylene-vinyl alcohol copolymer; VOH = vinyl alcohol. Further research on efficient film formation for food packaging b Sothornvit and others (2003). c applications along with a better understanding on how film properKrochta (1997). ties are affected by protein and plasticizer molecular weight, comtwin-screw extruded sheets had less pinholes and a more compact, position, microstructure, and interactions is still needed. Improvements in protein separation techniques and equipment design will uniform structure (Wang and Padua 2002). For twin-screw extruded fatty acids-zein sheets, Ha and Padua contribute to better understanding and process control. (2001) reported that external surfaces of the zein sheets were covReferences ered with a flake-like material, which was attributed to fatty acid e migration to the surface. After this deposit was removed, SEM im- Ar as JA. 1992. Extrusion of food proteins. Crit Rev Food Sci 32(4):36592. Cunningham P, Ogale AA, Dawson PL, Acton JC. 2000. Tensile properties of soy ages showed entanglements of thin zein fibers. protein isolate films produced by a thermal compaction technique. J Food Sci 65(4):66871. Film microstructural characteristics are closely related to the Cuq B, Gontard N, Guilbert S. 1997a. Thermal properties of fish myofibrillar proteinmechanical and barrier properties of the films, which in turn will based films as affected by moisture content. Polymer 38(10):2399405. Cuq B, Gontard N, Guilbert S. 1997b. Thermoplastic properties of fish myofibrillar determine film practical applications.
Table 5 --- Comparison of water vapor permeability of various protein-based lms.a
Film Applications
dible protein-based films obtained by thermoplastic processes provide the opportunity to fulfill the consumers demands and expectations of new food packaging systems that are convenient and environmentally friendly (Han 2005). Edible films can be used as wraps or formed into pouches, bags, casings, or sachets, resulting in reduced waste and improved recyclability of the packaging system. Wheat gluten and soy protein films have been explored as a replacement for collagen in sausage casings. Soy protein films have also been considered in the production of water-soluble pouches (Krochta 1997). Corn protein-based thermoplastic materials could be used for compostable items (di Gioia and Guilbert 1999). Proposed uses for whey protein-based films include formation of edible or biodegradable wraps for food products (Sothornvit and others 2007) and small pouches for preweighed ingredients (Hernandez-Izquierdo 2007). Addition of antimicrobials, antioxidants, and flavors might result in a wider range of applications.
Conclusions
hermoplastic processing of proteins for film formation has promising potential for large-scale production of edible films. Such films could be used for food wraps, layers between food components, or heat-sealed to form sacks, sachets, pouches, or bags to contain dry foods or preweighed ingredients. The films could also work together with conventional packaging to improve food quality, while reducing the amount and complexity of the packaging waste and making the package system more easily recyclable. The processability of the proteins depends on their transition from the glassy to the rubbery and free-flow states. These transitions are achieved through adequate use of plasticizers and appropriate processing conditions such as temperature and dwell time for compression molding, and feed rates, barrel temperature profile, screw configuration, and screw speed in the case of extrusion. Improvement of mechanical and barrier properties of protein films that would more closely resemble those of synthetic packJOURNAL OF FOOD SCIENCEVol. 73, Nr. 2, 2008
proteins: application to biopackaging fabrication. Polymer 38(16):40718. di Gioia L, Guilbert S. 1999. Corn protein-based thermoplastic resins: effect of some polar and amphiphilic plasticizers. J Agric Food Chem 47:125461. Fichtali J, van de Voort FR. 1989. Fundamental and practical aspects of twin screw extrusion. Cereal Food World 34(11):9218. Fitzsimons SM, Mulvihill DM, Morris ER. 2007. Denaturation and aggregation processes in thermal gelation of whey proteins resolved by differential scanning calorimetry. Food Hydrocolloid 21:63844. Ha TT, Padua GW. 2001. Effect of extrusion processing on properties of zein-fatty acids sheets. T ASAE 44(5):12238. Han JH. 2005. New technologies in food packaging: overview. In: Han JH, editor. Innovations in food packaging. San Diego, Calif.: Elsevier Academic Press. p 311. Hauck BW, Huber GR. 1989. Single screw vs twin screw extrusion. Cereal Food World 34(11):9309. Hernandez-Izquierdo VM. 2007. Thermal transitions, extrusion, and heat-sealing of whey protein edible films [dissertation]. Davis, Calif.: Univ. of California. 110 p. Jane J, Wang S, inventors; Iowa State University Research Foundation Inc., Ames, Iowa, assignee. 1996 June 4. Soy protein-based thermoplastic composition for preparing molded articles. U.S. patent 5,523,293. Jane J, Zhang SS, inventors; Iowa State University Research Foundation Inc., Ames, Iowa, assignee. 1998 Jan 20. Soy protein-based thermoplastic composition for foamed articles. U.S. patent 5,710,190. Khorshid N, Hossain M, Farid MM. 2007. Precipitation of food protein using high pressure carbon dioxide. J Food Eng 79:121420. Khwaldia K, Perez C, Banon S, Desobry S, Hardy J. 2004. Milk proteins for edible films and coatings. Crit Rev Food Sci 44:23951. Kokini JL, Cocero AM, Madeka H, de Graaf E. 1994. The development of state diagrams for cereal proteins. Trends Food Sci Technol 5:2818. Kristo E, Biliaderis G, Zampraka A. 2007. Water vapor barrier and tensile properties of composite caseinate-pullulan films: biopolymer composition effects and impact of beeswax lamination. Food Chem 101:75364. Krochta JM. 1997. Edible protein films and coatings. In: Damodaran S, Paraf A, editors. Food proteins and their applications. New York: Marcel Dekker Inc. p. 52950. Krochta JM. 2002. Proteins as raw materials for films and coatings: definitions, current status, and opportunities. In: Gennadios A, editor. Protein-based films and coatings. Boca Raton, Fla.: CRC Press. p 131. Krochta JM, De Mulder-Johnston C. 1997. Edible and biodegradable polymer films: challenges and opportunities. Food Technol-Chicago 51(2):6174. Krochta JM, Baldwin EA, Nisperos-Carriedo MO. 1994. Edible coatings and films to improve food quality. Lancaster, Pa.: Technomic Publication Co. 379 p. Lacroix M, Cooksey K. 2005. Edible films and coatings from animal-origin proteins. In: Han JH, editor. Innovations in food packaging. San Diego, Calif.:Elsevier Academic Press. p 30117. Li M, Lee TC. 1996. Effect of cysteine on the functional properties and microstructures of wheat flour extrudates. J Agric Food Chem 44(7):187180. Liu L, Kerry JF, Kerry JP. 2006. Effect of food ingredients and selected lipids on the physical properties of extruded edible films/casings. Int J Food Sci Technol 41:295 302. McMurry J. 1994. Quimica organica. Mexico: grupo editorial iberoamerica.1278 p. Moraru CI, Kokini JL. 2003. Nucleation and expansion during extrusion and microwave heating of cereal foods. Compr Rev Food Sci F 2:12038.
R38
Protein processing for lm formation . . .
Naga M, Kirihara S, Tokugawa Y, Tsuda F, Saito T, Hirotsuka M, inventors; Fuji Oil Co. Ltd. Osaka-fu, Japan, assignee. 1996 Oct 29. Process for producing edible proteinaceous film. U.S. patent 5,569,482. Ogale AA, Cunningham P, Dawson PL, Acton JC. 2000. Viscoelastic, thermal and microstructural characterization of soy protein isolate films. J Food Sci 65(4):6729. Perez-Gago MB, Krochta JM. 2005. Emulsion and bi-layer edible films. In: Han JH, editor. Innovations in food packaging. San Diego, Calif.: Elsevier Academic Press. p 384402. Pol H, Dawson P, Acton J, Ogale A. 2002. Soy protein isolate/corn-zein laminated films: transport and mechanical properties. J Food Sci 67(1):2127. Pommet M, Redl A, Morel MH, Domenek S, Guilbert S. 2003. Thermoplastic processing of protein-based bioplastics: chemical engineering aspects of mixing, extrusion and hot molding. Macromol Symp 197:20717. Pommet M, Redl A, Guilbert S, Morel MH. 2005. Intrinsic influence of various plasticizers on functional properties and reactivity of wheat gluten thermoplastic materials. J Cereal Sci 42:8191. Rakotonirainy AM, Padua GW. 2001. Effects of lamination and coating with drying oils on tensile and barrier properties of zein films. J Agric Food Chem 49(6):28603. Redl A, Morel MH, Bonicel J, Vergnes B, Guilbert S. 1999a. Extrusion of wheat gluten plasticized with glycerol: influence of process conditions on flow behavior, rheological properties, and molecular size distribution. Cereal Chem 76(3): 36170. Redl A, Morel MH, Bonicel J, Guilbert S, Vergnes B. 1999b. Rheological properties of gluten plasticized with glycerol: dependence on temperature, glycerol content and mixing conditions. Rheol Acta 38:31120. Robertson GL. 1993. Food packaging. Principles and practice. New York: Marcel Dekker Inc. 676 p. Sothornvit R, Krochta JM. 2000. Plasticizer effect on oxygen permeability of lactoglobulin films. J Agric Food Chem 48(12):6298302. Sothornvit R, Krochta JM. 2001. Plasticizer effect on mechanical properties of lactoglobulin films. J Food Eng 50:14955. Sothornvit R, Krochta JM. 2005. Plasticizers in edible films and coatings. In: Han JH, editor. Innovations in food packaging. San Diego, Calif.: Elsevier Academic Press. p 40333. Sothornvit R, Olsen CW, McHugh TH, Krochta JM. 2003. Formation conditions, watervapor permeability, and solubility of compression-molded whey protein films. J Food Sci 68(6):19859. Sothornvit R, Olsen CW, McHugh TH, Krochta JM. 2007. Tensile properties of compression-molded whey protein sheets: determination of molding condition and glycerol-content effects and comparison with solution-cast films. J Food Eng 78(3):85560. Stevens MP. 1999. Polymer chemistry. An introduction. New York: Oxford Univ. Press. 551 p. Talens P, Krochta JM. 2005. Plasticizing effects of beeswax and carnauba wax on tensile and water vapor permeability properties of whey protein films. J Food Sci 70(3):E23943. Tolstoguzov VB. 1993. Thermoplastic extrusionthe mechanism of the formation of extrudate structure and properties. J Am Oil Chem Soc 70(4):41724. Wang Y, Padua GW. 2002. Zein-based biodegradable packaging films produced by extrusion [poster]. In: IFT Annual Meeting and Food Expo; 2002 June 1619; Anaheim, Calif. Chicago, Ill.: Institute of Food Technologists. Poster nr 100B37. Zhang J, Mungara P, Jane J. 2001. Mechanical and thermal properties of extruded soy protein sheets. Polymer 42:256978.
Vol. 73, Nr. 2, 2008JOURNAL OF FOOD SCIENCE
R39
R: Concise Reviews and in Food Science
Das könnte Ihnen auch gefallen
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (587)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (73)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2219)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (265)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (119)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- QAQC Inspection Services Technical Proposal SummaryDokument69 SeitenQAQC Inspection Services Technical Proposal SummaryMathias OnosemuodeNoch keine Bewertungen
- Airfix 2011 CatalogueDokument132 SeitenAirfix 2011 CatalogueGordon Sorensen0% (1)
- NFPA 99 Risk AssessmentDokument5 SeitenNFPA 99 Risk Assessmenttom ohnemusNoch keine Bewertungen
- BR18 Mechanical Engineering Robotics Semester VIDokument2 SeitenBR18 Mechanical Engineering Robotics Semester VIPRAVEeNoch keine Bewertungen
- Kooltherm PipeDokument8 SeitenKooltherm Pipenaseema1Noch keine Bewertungen
- BS 00011-2015Dokument24 SeitenBS 00011-2015fazyroshan100% (1)
- Grid Xtreme VR Data Sheet enDokument3 SeitenGrid Xtreme VR Data Sheet enlong bạchNoch keine Bewertungen
- Banaue Rice Terraces - The Eighth WonderDokument2 SeitenBanaue Rice Terraces - The Eighth Wonderokloy sanchezNoch keine Bewertungen
- Conflict of Laws (Summary Paper)Dokument13 SeitenConflict of Laws (Summary Paper)Anonymous CWcXthhZgxNoch keine Bewertungen
- Portfolio Corporate Communication AuditDokument8 SeitenPortfolio Corporate Communication Auditapi-580088958Noch keine Bewertungen
- Soal Pat Inggris 11Dokument56 SeitenSoal Pat Inggris 11dodol garutNoch keine Bewertungen
- The New Breed of Rally BDA: Motor SportDokument8 SeitenThe New Breed of Rally BDA: Motor SportHarold MorleyNoch keine Bewertungen
- JAM 2020 Information Brochure: Admission to M.Sc., Joint M.Sc.-Ph.D., M.Sc.-Ph.D. Dual Degree and Integrated Ph.D. ProgrammesDokument51 SeitenJAM 2020 Information Brochure: Admission to M.Sc., Joint M.Sc.-Ph.D., M.Sc.-Ph.D. Dual Degree and Integrated Ph.D. ProgrammesVaibhav PachauleeNoch keine Bewertungen
- Csit 101 Assignment1Dokument3 SeitenCsit 101 Assignment1api-266677293Noch keine Bewertungen
- WebquestDokument3 SeitenWebquestapi-501133650Noch keine Bewertungen
- 2014 Chevrolet Cruze maintenance schedule guideDokument2 Seiten2014 Chevrolet Cruze maintenance schedule guidericardo rodriguezNoch keine Bewertungen
- Engineering Ethics in Practice ShorterDokument79 SeitenEngineering Ethics in Practice ShorterPrashanta NaikNoch keine Bewertungen
- Investigations in Environmental Science: A Case-Based Approach To The Study of Environmental Systems (Cases)Dokument16 SeitenInvestigations in Environmental Science: A Case-Based Approach To The Study of Environmental Systems (Cases)geodeNoch keine Bewertungen
- Supply AnalysisDokument5 SeitenSupply AnalysisCherie DiazNoch keine Bewertungen
- Machine Problem 6 Securing Cloud Services in The IoTDokument4 SeitenMachine Problem 6 Securing Cloud Services in The IoTJohn Karlo KinkitoNoch keine Bewertungen
- Practice Questions & Answers: Made With by SawzeeyyDokument141 SeitenPractice Questions & Answers: Made With by SawzeeyyPhake CodedNoch keine Bewertungen
- Fayol's Principles in McDonald's ManagementDokument21 SeitenFayol's Principles in McDonald's Managementpoo lolNoch keine Bewertungen
- Power Efficiency Diagnostics ReportDokument16 SeitenPower Efficiency Diagnostics Reportranscrib300Noch keine Bewertungen
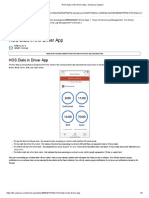
- HOS Dials in The Driver App - Samsara SupportDokument3 SeitenHOS Dials in The Driver App - Samsara SupportMaryNoch keine Bewertungen
- Audit Report of CompaniesDokument7 SeitenAudit Report of CompaniesPontuChowdhuryNoch keine Bewertungen
- Panameterics GF 868 Flare Gas Meter PDFDokument8 SeitenPanameterics GF 868 Flare Gas Meter PDFDaniel DamboNoch keine Bewertungen
- Dani RodrikDokument12 SeitenDani Rodrikprogramas4242Noch keine Bewertungen
- Gilette Case - V3Dokument23 SeitenGilette Case - V3Vidar Halvorsen100% (3)
- Summer Training Report On HCLDokument60 SeitenSummer Training Report On HCLAshwani BhallaNoch keine Bewertungen
- NSTP 1: Pre-AssessmentDokument3 SeitenNSTP 1: Pre-AssessmentMaureen FloresNoch keine Bewertungen