Beruflich Dokumente
Kultur Dokumente
L-16 Purine Biochemistry and Uric Acid Metabolism - XML
Hochgeladen von
Julia HangaOriginalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
L-16 Purine Biochemistry and Uric Acid Metabolism - XML
Hochgeladen von
Julia HangaCopyright:
Verfügbare Formate
Lecture 16.
Purine Biochemistry and Uric Acid Metabolism
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
McGill Department of Medicine Division of Rheumatology Montreal General Hospital 937-6011 loc. 4178 M. Starr michael.starr@muhc.mcgill.ca
Objectives
The objectives of these lectures and this handout are for you to learn: 1. Basic clinical concepts of urate crystal deposition disease. 2. Basic biochemistry of purine compounds: the substrates and major enzymes involved in pathways of purine metabolism and uric acid formation. 3. Basic physicochemical properties of urate and uric acid. 4. The mechanisms of urate synthesis and elimination. 5. To understand the mechanisms that lead to hyperuricemia and how dietary modification or drugs could be used to restore normouricemia.
I. Gout
HISTORY
Excellent descriptions of gout can be found in the earliest medical writings. The aphorisms of Hippocrates documented the fact that gout is predominantly a disease of adult men and only appears in women after the menopause. Sydenham was himself a gout sufferer and his vivid descriptions of the disease, written in 1683 can still make the big toe itch! Gout was extremely common in Georgian England, and many of the best known figures in English political and cultural life suffered from the disease; the extreme pain of acute attacks of gout has been responsible for anger, frustration, and misjudged decisions in the highest political circles (e.g. Pitt and the Boston Tea Party).
DEFINITION
Gout a term first used in the 13th century A.D. is derived from the Latin 'gutta' (a drop) which reflects the notion that gout resulted from a local instillation of a malevolent humour. We have since recognized that gout is a crystal deposition disease; its clinical and pathological manifestations are due to the presence of crystalline salts of urate within the affected tissues.
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
CLINICAL FEATURES
Hyperuricemia: a serum or plasma urate concentration greater than 7.0 mg/dl (0.42 mmol/l) in males and 6.0 mg/dl (0.36 mmol/l) in females. Acute gouty arthritis: sudden, intense inflammation in and ``around the joints (most typically, but not limited to, the big toe) and other areas of soft tissues such as the Achilles tendon (heel), olecranon bursa (elbow), helix of the ear. Urolithiasis: uric acid stones in the kidney and urinary collecting system. Chronic interstitial nephropathy: disease related to the deposition of monosodium urate monohydrate crystals in the substance of the kidney itself.
EPIDEMIOLOGY OF GOUT AND HYPERURICEMIA
Gout is predominantly a disease of adult males, with a peak incidence in the fifth decade. The prevalence in males varies from about 10-20/1,000 and in females 1-6/1,000. It is the second most common cause of inflammatory arthritis in males over the age of 30 in the U.S.A. It causes significant short-term disability, occupational limitation, and utilization of medical services. Hyperuricemia is demonstrable in at least 5% of asymptomatic American (and probably Canadian) men on at least one occasion during adulthood. The risk of developing clinically apparent urate crystal deposition disease increases as the level of serum uric acid rises.
II. Purine Metabolism
INTRODUCTION
Recognition of the role of purines in human disease began with the observation that uric acid, a purine base, was a component of some renal calculi and in the form of monosodium urate, was a major constituent of tophi (deposits of urate in soft tissues), was elevated in the serum of gouty patients, and was present, in crystalline form, in synovial (joint) fluid during the acute attack of gouty arthritis. In humans, uric acid is derived both from the ingestion of foods containing purines and from the endogenous synthesis of purine nucleotides, which are building blocks in the synthesis of nucleic acids.
FIG. 1. PURINE BIOCHEMISTRY
The parent compound, the purine base, is composed of a six-membered pyrimidine ring fused to the five- membered imidazole ring.
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Fig. 1 The most important purines are: adenine guanine hypoxanthine xanthine uric acid The purines all show lactam-lactim isomerism and may be written in either form (Fig. 2).
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
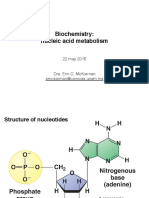
Fig. 2 Purine nucleosides are composed of a purinebase plus a pentose joined to the base by a -Nglycosyl bond between carbon atom 1 of the pentose and nitrogen atom 9 of the purine base. There are two series of nucleosides: ribonucleosides, which contain D-ribose as the sugar component deoxyribonucleosides, which contain 2-deoxyribose (Fig 3)
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Fig. 3 Purine nucleotides and deoxynucleotides consist of a nucleoside or a deoxynucleoside with a phosphate group in ester linkage with carbon 5 of the pentose (Fig. 4).
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Fig. 4 The nucleosides and deoxynucleosides may exist as: 5'-monophosphates 5'-diphosphates 5'-triphosphates The phosphoric acid residues of these compounds are designated by the symbols a, , (Fig. 5).
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Fig. 5 These compounds serve: 1. as building blocks for RNA and DNA 2. as precursors of the cyclic nucleotides, adenosine 3',5'-cyclic phosphate, and guanosine 3',5'-cyclic phosphate 3. as a source of chemical energy 4. as precursors of various purine cofactors and coenzymes such as nicotinamide adenine dinucleotide (NAD)
PURINE METABOLISM (Fig. 6)
The synthesis of purine nucleotides involves alternative biochemical pathways that are closely regulated. In the pathway of purine synthesis de novo, a purine ring is synthesized from small molecule precursors of uric acid, sequentially added to a ribose-phosphate backbone donated by 5 -phophoribosyl 1-pyrophosphate (PRPP). The first reaction committed to the pathway, catalyzed by the enzyme amido phosphoribosyltransferase (reaction 1 in Fig.), is the major site of the regulation of this pathway by means of an antagonistic interaction between inhibition by purine nucleotide products and activation by PRPP, a substrate usually present in limited concentrations in the cell. Additional
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
sites of control of purine nucleotide production have been identified at the level of the PRPP synthesis (reaction 3) and at the distal branch point governing distribution of newly formed nucleotides into adelylate and guanylate derivatives. The alternative pathway of purine nucleotide synthesis involves two enzymes, adenine phosphoribosyltransferase (reaction 4) and hypoxanthine-guanine phosphoribosyltransferase (HGPRT, reaction 2), which catalyze reactions between PRPP and the respective purine base substrates in the single-step synthesis of purine nucleotides. Among the factors governing the relationship between rates of purine base salvage and purine synthesis de novo are the availability of PRPP and the concentrations of the nucleotide products common to both pathways. The catabolicsteps that generate uric acid from nucleic acids and free purine nucleotides involve degradation through purine nucleoside intermediates to hypoxanthine and xanthine. The latter are ultimately oxidized to uric acid in sequential reactions catalyzed by the enzyme xanthine oxidase (reaction 8).
Fig. 6
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
III. Physicochemical Properties of Uric Acid and Urate
Uric acid is a weak acid which is ionized at normal body pH and thus occurs in the blood or tissues in the form of the urate ion. When ionized, uric acid can form salts with various cations but 98% of extracellular uric acid is in the form of the monosodium urate. However the proportion in the form of uric acid or urate is pH dependent so that the ratio between these two forms may vary considerably in urine. Effect of pH on Uric Acid
Fig. 7 At pH 5.7 equal amounts of uric acid and urate are present in solution. The solubility of urate and uric acid is critical to the development of crystals. Urate is considerably more soluble in plasma, synovial fluid and urine than in aqueous solutions. Even so, above a concentration of about 7.0 mg/dl (0.42 mmol/l), plasma is supersaturated with urate. Perhaps because this is often a stable situation and because of the other constituents of plasma, there may be no tendency to crystal formation. The solubility of uric acid in urine rises
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
exponentially as the pH increases above 4. However, there is little change in the solubility of urate within the pH range that may exist in the plasma, synovial fluid and other tissues. Both urate and uric acid solubility fall with decreasing temperatures (Fig. 7).
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
IV. Urate Synthesis and Elimination
Urate is derived from purines that may be ingested or synthesized from ingested foods as well as being reutilized following cell breakdown. Urate is then eliminated via the kidneys and alimentary tract. (Fig. 8).
Fig. 8
URATE PRODUCTION
The pathways of production of uric acid were outlined in Fig. 6 and in a simpler form in Fig. 13. Urate production is therefore dependent upon purine nucleotide metabolism and the function of xanthine oxidase. Whereas there are several mechanisms for the reutilization of purine bases to nucleosides and nucleotides, degradation of these purine bases by xanthine oxidase is irreversible.
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Purine availability can be altered by several factors as illustrated by Fig. 8. Purines can be derived directly from the diet or by synthesis from small molecular precursors. Purine bases derived from tissue nucleic acids may also be reutilized. These arise from normal cell turnover but the amounts can be greatly increased in proliferative disorders of the bone marrow, particularly hematologic malignancies and by cytotoxic therapy.
URATE ELIMINATION
In order to maintain homeostasis, urate must be eliminated from the body as there is no uricase and thus no metabolism of urate in human tissues. Approximately 2/3 of produced urate is eliminated via the kidneys with the remainder being excreted into the alimentary tract. It is probable that the elimination of urate via the alimentary tract is a passive process and varies with plasma urate concentrations. Under normal conditions, negligible amounts of urate are found in the feces because it is degraded by colonic bacteria. In a sterilized bowel, on the other hand, urate excreted into the lumen does not undergo uricolysis and may be found in the feces. Almost all of the urate in the plasma is filtered at the glomerulus. Although there is some binding of urate to plasma proteins, this is of low affinity in vivo, readily reversible and not of physiologic significance in man. Following filtration, there is almost complete reabsorption of urate in the proximal tubule such that only a small amount of the filtered urate passes to the loop of Henle. Thus, in proximal tubular disorders such as the Fanconi syndrome or acquired nonselective proximal tubular transport defects, a failure of urate reabsorption resulting in hyperuricosuria and hypouricemia may occur.
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Fig. 9 Perhaps the most important mechanism for maintaining normal renal urate excretion is the active tubular secretion of urate. This may account for as much as 50% of the urate which is filtered. Neither the site nor mechanism for this urate secretion has been precisely identified in man. Lactate, -hydroxybutyrate and, acetoacetate are thought to decrease urate excretion by inhibiting tubular secretion of urate. The variation in the amount of urate that is finally excreted by the kidney is most likely due to alterations in the reabsorption of secreted urate. The precise site of postsecretory reabsorption in man is uncertain, as is whether it occurs distally to or in the same segment as urate secretion. In all, approximately 10% of filtered urate is finally excreted in the urine. Clearly, abnormalities at any of the four stages of renal urate excretion may have a profound effect on urate homeostasis. (Fig 9).
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
V. Etiology of Hyperuricemia
Hyperuricemia may result from overproduction or underexcretion of urate. In overproduction of urate, the purine precursors may be of endogenous or exogenous (i.e. dietary) origin. With respect to underexcretion of urate, this depends principally upon renal handling of urate. Overall, the great majority of patients with gout and hyperuricemia demonstrate urate underexcretion as judged by a urate clearance of less than 6 ml/min or a urate to creatinine clearance ratio of less than 6%. However, in practical terms, the combination of a relative excess of dietary purine consumption together with urate underexcretion is likely to be the basis of hyperuricemia in many patients with gout. Primary hyperuricemia refers to those circumstances in which elevated serum urate levels or manifestations of urate deposition are due to inherently disordered uric acid metabolism not associated with another acquired disorder and in which gout is a prominent feature of the clinical picture. Secondary hyperuricemia refers to circumstances in which gout is a minor clinical fearure secondary to any of a number of genetic or acquired processes.
URATE OVERPRODUCTION
About 10% of patients with hyperuricemia are overproducers of uric acid of which no more than 10% (i.e. 1% of all hyperuricemic people) have primary identifiable inherited derangements in mechanisms regulating purine nucleotide synthesis de novo. In both partial deficiency of HGPRT and milder forms of superactivity of PRPP synthetase, early adult- onset gout and a high incidence of uric acid urinary tract stones constitute the usual clinical phenotype. Severe HGPRT deficiency is associated with spasticity, choreoathetosis, mental retardation, and compulsive self-mutilation (Lesch-Nyhan Syndrome). Lesser neurologic lesions may be found in a minority of patients with partial HGPRT deficiency. Intracellular accumulation of PRPP is a result of diminished utilization of this regulatory substrate in purine base salvage. This drives purine synthesis de novo at an increased rate in HGPRT deficiency. In addition, in the absence of HGPRT, hypoxanthine, once formed, cannot be reutilized and can only be degraded. In the case of variant forms of PRPP synthetase with excessive activity, increased PRPP availability for purine synthesis results from increased rates of PRPP generation. Both of these enzymes are produced from X-linked genes, and thus heterozygous men are affected. Hyperuricemia detected in prepubertal boys suggests that one of these enzymatic defects could be the cause. An increase in the net intracellular degradation of the adenine nucleotide, ATP, has been proposed as a common thread linking hyperuricemia in several conditions which are listed in Fig. 11, below. In particular, the accelerated degradation of ATP to AMP via the conversion of acetate to acetyl CoA in the metabolism of ethanol appears to be a critical mechanism in causing overproduction of uric acid and hyperuricemia associated with alcohol ingestion (Fig. 10).
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Fig. 10 Click here for the Flash Animation Fig. 11. Classification of hyperuricemia
Uric acid overproduction
Primary hyperuricemia
Uric acid underexcretion
Primary hyperuricemia
1. 2. 3.
Idiopathic HGPRT deficiency (partial complete) PRPP synthetase superactivity
and
Idiopathic
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Secondary hyperuricemia
Secondary hyperuricemia
1. 2.
3.
Excessive dietary purine intake Increased nucleotide turnover (e.g. myeloproliferative and lymphoproliferative disorders, hemolytic disorders, psoriasis) Accelerated ATP degradation glycogen storage diseases (Types I, III, V, VII) fructose ingestion, hereditary fructose intolerance hypoxemia and tissue underperfusion severe muscle exertion alcohol abuse
1. 2. 3. 4.
Diminished renal function Inhibition of tubular urate secretion competitive anions (e.g. keto- and lactic acidosis) Enhanced tubular urate reabsorption dehydration, diuretics Mechanism incompletely defined hypertension hyperparathyroidism certain drugs (e.g. low dose aspirin, many diuretics, lead nephropathy
Fig. 12
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Fig. 13 Source of figures and tables: Figures 76.6, 76.7, 76.8, 76.9, 76.10, 76.11, 76.17 and Table 76.10 are from William N. Kelly and H. Ralph Schumacher, Jr. "Crystal-Associated Synovitis" from Textbook of Rheumatology. W.N. Kelley, E.D. Harris, S. Ruddy, C.B. Sledge editors, W.B. Saunders, Philadelphia, U.S.A., 1993, pp1291-1336. Figures 18.1, 18.2, 18.3 are from "Gout" from Atlas of Clinical Rheumatology. P.A. Dieppe, P.A. Bacon, A.N. Bamji, I. Watt editors, Gower Medical Publishing Ltd, London, England, 1986, p 18.2. Figure 31A-1 and Table 31A-1 are from Robert Terkeltaub "Gout" from Primer on the Rheumatic Diseases, H.R. Schumacher, Jr., J.H. Klippel, W.J. Koopman editors, The Arthritis Foundation, Atlanta, U.S.A. 1993, pp209-213.
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Lecture 16. Purine Biochemistry and Uric Acid Metabolism
Das könnte Ihnen auch gefallen
- Regulation of Uric Acid Metabolism and Excretion PDFDokument7 SeitenRegulation of Uric Acid Metabolism and Excretion PDFYulanda YomemotoNoch keine Bewertungen
- Current Concepts of And: Hyperuricemia GoutDokument13 SeitenCurrent Concepts of And: Hyperuricemia GoutAnonymous h0XxWy8SNoch keine Bewertungen
- Amino Acid Protein and Nucleic Acid Metabolism 20182019 LectureDokument18 SeitenAmino Acid Protein and Nucleic Acid Metabolism 20182019 LectureMwanja MosesNoch keine Bewertungen
- Maiuolo 2016Dokument7 SeitenMaiuolo 2016okaNoch keine Bewertungen
- Written Report 10Dokument5 SeitenWritten Report 10Sherma Sheikh karimNoch keine Bewertungen
- Nucleotide MetabolismDokument29 SeitenNucleotide MetabolismThe SpiritsNoch keine Bewertungen
- Uric Acid MetabolismDokument3 SeitenUric Acid MetabolismAlifah SyarafinaNoch keine Bewertungen
- BP U9e Metabolism of Nucleic AcidsDokument49 SeitenBP U9e Metabolism of Nucleic AcidsChristian Angelo AgbunagNoch keine Bewertungen
- Metabolism of Nucleoproteins Part IDokument50 SeitenMetabolism of Nucleoproteins Part IAgafioNoch keine Bewertungen
- UrinalysisDokument10 SeitenUrinalysisMichelle100% (2)
- DR Okunowo Wahab Introductory Molecular Biology Lecture Note I (Nucleotides Metabolism)Dokument20 SeitenDR Okunowo Wahab Introductory Molecular Biology Lecture Note I (Nucleotides Metabolism)modelprof100% (2)
- Biochem Metabolismo Ácidos NucléicosDokument27 SeitenBiochem Metabolismo Ácidos NucléicosLidia Escutia GuadarramaNoch keine Bewertungen
- Purine Synthesis °radation-GoutDokument51 SeitenPurine Synthesis °radation-Goutmohammed aliNoch keine Bewertungen
- Heme Metabolism PDFDokument19 SeitenHeme Metabolism PDFAnonymous jW7BU44ACNoch keine Bewertungen
- Chronic Hyperuricemia, Uric Acid Deposit and Cardiovascular RiskDokument7 SeitenChronic Hyperuricemia, Uric Acid Deposit and Cardiovascular RiskBelleNoch keine Bewertungen
- Clinical BiochemistryDokument73 SeitenClinical BiochemistryEslam NassarNoch keine Bewertungen
- On Digestive Proteolysis: Being the Cartwright Lectures for 1894Von EverandOn Digestive Proteolysis: Being the Cartwright Lectures for 1894Noch keine Bewertungen
- The Metabolism of Propionic AcidDokument11 SeitenThe Metabolism of Propionic AcidMUTHONI IRERINoch keine Bewertungen
- UlcerDokument10 SeitenUlcerDahniar EndahfuriNoch keine Bewertungen
- Determination of Uric AcidDokument4 SeitenDetermination of Uric AcidHasan ShahriarNoch keine Bewertungen
- Mariotti, AJCN, 2013Dokument8 SeitenMariotti, AJCN, 2013Dianne Faye ManabatNoch keine Bewertungen
- Buzanovskii 2015Dokument84 SeitenBuzanovskii 2015huynh ngoc linhNoch keine Bewertungen
- Uric Acid Determination PDFDokument10 SeitenUric Acid Determination PDFJhon Jerome DapdapNoch keine Bewertungen
- BSC Sem VI Nuc Aid MetabDokument24 SeitenBSC Sem VI Nuc Aid Metabstarboigaming08Noch keine Bewertungen
- ChoiDokument19 SeitenChoiLuciana RafaelNoch keine Bewertungen
- Nutrients: Fructose Intake, Serum Uric Acid, and Cardiometabolic Disorders: A Critical ReviewDokument15 SeitenNutrients: Fructose Intake, Serum Uric Acid, and Cardiometabolic Disorders: A Critical ReviewAngely NovianaNoch keine Bewertungen
- Non-Protein Nitrogen CompoundsDokument6 SeitenNon-Protein Nitrogen CompoundspixiedustNoch keine Bewertungen
- How Orotates WorkDokument3 SeitenHow Orotates WorkAnonymous 75JL8fvYb100% (1)
- Purine Metabolism PDFDokument29 SeitenPurine Metabolism PDFtrinitysugumar0% (1)
- NPNs and Kidney Function TestsDokument10 SeitenNPNs and Kidney Function TestsAngelo Jude CobachaNoch keine Bewertungen
- Phenylketonuria: An Inborn Error of Phenylalanine MetabolismDokument11 SeitenPhenylketonuria: An Inborn Error of Phenylalanine MetabolismAndreea StefanNoch keine Bewertungen
- Omcl2015 813737Dokument7 SeitenOmcl2015 813737fadimeatesNoch keine Bewertungen
- Cutanea Tarda PorphyriaDokument9 SeitenCutanea Tarda PorphyriaKanwal RashidNoch keine Bewertungen
- J. Biol. Chem.-1957-Busch-377-87Dokument12 SeitenJ. Biol. Chem.-1957-Busch-377-87Aiims2k18 UG mbbsNoch keine Bewertungen
- Acid-Base Homeostasis: Clin J Am Soc Nephrol. 10.2215/CJN.07400715 26597304Dokument18 SeitenAcid-Base Homeostasis: Clin J Am Soc Nephrol. 10.2215/CJN.07400715 26597304Christine SiraitNoch keine Bewertungen
- Thyroid Hormone Deficiency Alters Expression of AcDokument13 SeitenThyroid Hormone Deficiency Alters Expression of AcNatty NigussieNoch keine Bewertungen
- The Measurement of Total Serum Proteins by The Biuret MethodDokument5 SeitenThe Measurement of Total Serum Proteins by The Biuret MethodsivaNoch keine Bewertungen
- Metabolic Changes of DrugsDokument103 SeitenMetabolic Changes of DrugsDaniel Wang100% (2)
- Naturally Occurring Peptide Hormones and Neurotransmitters, Synthesis ofDokument30 SeitenNaturally Occurring Peptide Hormones and Neurotransmitters, Synthesis ofazzaassNoch keine Bewertungen
- Orotic Acid Excretion and Arginine MetabolismDokument6 SeitenOrotic Acid Excretion and Arginine MetabolismKevin Hernandez PerezNoch keine Bewertungen
- Minerals C 4Dokument40 SeitenMinerals C 4Jasmine sindhooNoch keine Bewertungen
- Reaction Paper ON Uric Acid and Ammonia: Introduction To Medical LaboratoryDokument3 SeitenReaction Paper ON Uric Acid and Ammonia: Introduction To Medical LaboratoryJamil B. AsumNoch keine Bewertungen
- Nitro Science White Paper-04 13-En-AllDokument11 SeitenNitro Science White Paper-04 13-En-Allapi-302657321Noch keine Bewertungen
- Larussa 2017Dokument25 SeitenLarussa 2017alan.rangel.puenteNoch keine Bewertungen
- Amino Acid Metabolism II 10-15-08Dokument35 SeitenAmino Acid Metabolism II 10-15-08Timothy George100% (1)
- FrancesFranciscoCC2 1Dokument6 SeitenFrancesFranciscoCC2 1Frances FranciscoNoch keine Bewertungen
- Biochemistr Y: Digital Assignment 2Dokument15 SeitenBiochemistr Y: Digital Assignment 2Ann Angella sibyNoch keine Bewertungen
- Go To:: 1.1. Uric Acid FormationDokument18 SeitenGo To:: 1.1. Uric Acid FormationMovies EntertainmentNoch keine Bewertungen
- Regulation of Fatty Acid and Glycerolipid Metabolism: FEBS 11th Meeting in Copenhagen 1977, Volume 46 Symposium A5Von EverandRegulation of Fatty Acid and Glycerolipid Metabolism: FEBS 11th Meeting in Copenhagen 1977, Volume 46 Symposium A5Raymond DilsNoch keine Bewertungen
- Acid BaseDokument10 SeitenAcid BaseOke RinaNoch keine Bewertungen
- Full Lab Report On: Exercise No. 4 Protein DenaturationDokument8 SeitenFull Lab Report On: Exercise No. 4 Protein DenaturationElaine FaloNoch keine Bewertungen
- L 3 Proteins Peptide Bond FormationDokument21 SeitenL 3 Proteins Peptide Bond FormationAhmed Zubair IrshadNoch keine Bewertungen
- High Catalytic Activity of Human Cytochrome P450 Co-Expressed With Human NADPH-Cytochrome P450 Reductase in Escherichia Coli (1998)Dokument11 SeitenHigh Catalytic Activity of Human Cytochrome P450 Co-Expressed With Human NADPH-Cytochrome P450 Reductase in Escherichia Coli (1998)Nimra Naveed ShaikhNoch keine Bewertungen
- Approved BiochemDokument4 SeitenApproved BiochemTempo RaryNoch keine Bewertungen
- Extracellular PH Regulates Bone Cell FunctionDokument4 SeitenExtracellular PH Regulates Bone Cell FunctionLeader AlfasonNoch keine Bewertungen
- RDS SurfactantDokument16 SeitenRDS SurfactantJulia HangaNoch keine Bewertungen
- BPJ 31 Antihypertensives Pages 14-21Dokument8 SeitenBPJ 31 Antihypertensives Pages 14-21Julia HangaNoch keine Bewertungen
- 133 Preg and Lactation Table WebDokument117 Seiten133 Preg and Lactation Table WebJulia HangaNoch keine Bewertungen
- Roxette - Crash! Boom! Bang!Dokument2 SeitenRoxette - Crash! Boom! Bang!Julia HangaNoch keine Bewertungen
- 2013 Esc CadDokument62 Seiten2013 Esc CadJulia HangaNoch keine Bewertungen
- Warfarin Flow SheetDokument1 SeiteWarfarin Flow SheetJulia HangaNoch keine Bewertungen
- Febuxostat ReviewDokument12 SeitenFebuxostat ReviewJulia HangaNoch keine Bewertungen
- WC500133437 PDFDokument24 SeitenWC500133437 PDFJulia HangaNoch keine Bewertungen
- Gout and Hyperuricemia: A Peer-Reviewed Bulletin For The Family PhysicianDokument6 SeitenGout and Hyperuricemia: A Peer-Reviewed Bulletin For The Family PhysicianRhoda LicardoNoch keine Bewertungen
- Gout BookDokument451 SeitenGout BookJulia Hanga50% (2)
- Review Shows EGb 761 Optimizes Standards of Life For Tinnitus PatientsDokument2 SeitenReview Shows EGb 761 Optimizes Standards of Life For Tinnitus PatientsTotoDodongGusNoch keine Bewertungen
- Radiology X-Rayfilm ScreensDokument39 SeitenRadiology X-Rayfilm ScreensFourthMolar.comNoch keine Bewertungen
- Marketing Sensing FrameworkDokument8 SeitenMarketing Sensing FrameworkHaseeb Ali0% (1)
- StreptokinaseDokument2 SeitenStreptokinasePramod RawoolNoch keine Bewertungen
- Theory and Practice of Addiction Counseling I1686Dokument16 SeitenTheory and Practice of Addiction Counseling I1686pollomaximus 444Noch keine Bewertungen
- Presentation 2017 ENGDokument20 SeitenPresentation 2017 ENGpabloNoch keine Bewertungen
- Bios Life Slim UAEDokument4 SeitenBios Life Slim UAEJey BautistaNoch keine Bewertungen
- Dan CV WordDokument4 SeitenDan CV WorddsnavarretteNoch keine Bewertungen
- SWS Bibliography1Dokument3 SeitenSWS Bibliography1Valaki MimiNoch keine Bewertungen
- Immune Thrombocytopenia (Second Option)Dokument16 SeitenImmune Thrombocytopenia (Second Option)Fein MalricNoch keine Bewertungen
- Kevin Chen - Book Review: Scientific Qigong Exploration: The Wonders and Mysteries of Qi by Lu, ZuyinDokument6 SeitenKevin Chen - Book Review: Scientific Qigong Exploration: The Wonders and Mysteries of Qi by Lu, ZuyinSonyRedNoch keine Bewertungen
- Harga Cap Lang 2018Dokument13 SeitenHarga Cap Lang 2018Ayo'e Harpotter's D'floreantzNoch keine Bewertungen
- Malunion Delayed Union and Nonunion FracturesDokument31 SeitenMalunion Delayed Union and Nonunion FracturesutisuryanjNoch keine Bewertungen
- Breast Cancer Brachytherapy - More ChoicesDokument5 SeitenBreast Cancer Brachytherapy - More ChoicesDr. Robert KuskeNoch keine Bewertungen
- Drug Price List - 25-05-2016Dokument636 SeitenDrug Price List - 25-05-2016shajbabyNoch keine Bewertungen
- Brief History of Makabali HospitalDokument1 SeiteBrief History of Makabali Hospitalpaulo_070390100% (1)
- Freedman Goldberg Reichmann The Radical Act of Inward LookingDokument35 SeitenFreedman Goldberg Reichmann The Radical Act of Inward LookingSantharaj KuppuswamyNoch keine Bewertungen
- NPI Reflects Spring 2013Dokument12 SeitenNPI Reflects Spring 2013npinashvilleNoch keine Bewertungen
- MAN ParaTitle-Manuscript - Grace-EpresDokument80 SeitenMAN ParaTitle-Manuscript - Grace-EpresSevered AppleheadNoch keine Bewertungen
- Case Presentation PBL 12 (Bipolar Disorder)Dokument65 SeitenCase Presentation PBL 12 (Bipolar Disorder)Rhomizal Mazali83% (6)
- NURSING CARE PLAN Impaired Transfer AbilityDokument1 SeiteNURSING CARE PLAN Impaired Transfer AbilityAce Dioso Tubasco100% (1)
- Xeo TreatmentsDokument13 SeitenXeo TreatmentsSimone WallisNoch keine Bewertungen
- ASCCP Management Guidelines - August 2014 PDFDokument24 SeitenASCCP Management Guidelines - August 2014 PDFAnita BlazevskaNoch keine Bewertungen
- 10 DOH Approved Herbal MedicationsDokument33 Seiten10 DOH Approved Herbal MedicationsBeanncaAngelesNoch keine Bewertungen
- NCP - Acute PainDokument3 SeitenNCP - Acute PainRoxanne NavarroNoch keine Bewertungen
- Oral Submucous Fibrosis Medical Management PDFDokument8 SeitenOral Submucous Fibrosis Medical Management PDFDame rohanaNoch keine Bewertungen
- Chapter 012Dokument5 SeitenChapter 012lukeNoch keine Bewertungen
- Sop Chlorine GasDokument3 SeitenSop Chlorine GashmtlionNoch keine Bewertungen
- Combination of Angkak (Red Yeast Rice), Red Guava (Psidium Guajava Linn) Leaf Extract and Red Guava Fruit Juice Increase Thrombocyte in Quinine-Exposed RatsDokument6 SeitenCombination of Angkak (Red Yeast Rice), Red Guava (Psidium Guajava Linn) Leaf Extract and Red Guava Fruit Juice Increase Thrombocyte in Quinine-Exposed RatsIOSR Journal of PharmacyNoch keine Bewertungen
- 01 - Onsite Sanitation TechnologiesDokument80 Seiten01 - Onsite Sanitation TechnologiesDivyam ShreevatsalNoch keine Bewertungen