Beruflich Dokumente
Kultur Dokumente
J Values Explnd
Hochgeladen von
Ari SinghOriginalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
J Values Explnd
Hochgeladen von
Ari SinghCopyright:
Verfügbare Formate
223
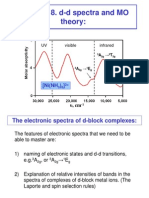
Consider the d-d electronic transitions for Transition Metal Complexes. Before we can do this, we must realize that spectroscopic transitions occur between states and not orbitals. Therefore: We must determine states for various electronic configurations in: 1. Free atoms or ions without a ligand set Term Symbols 2. Complexes in a crystal (or ligand field)
Electronic States and Multiplicities
(rather than orbital states and energies) are Relevant to Electronic Spectroscopy and EPR (Electron Paramagnetic Resonance) Spectroscopy
Free ion states
Free ion
versus
weak field ligands
Orbital States (ligand field)
strong field ligands
Determine term Symbols
to relate the two Determine Electronic use a Tanabe-Sugano States and Multiplicities Diagram
224
Free ions can be described by Term Symbols:
2S + 1
LJ
where: 2S + 1 is spin multiplicity L is Total orbital angular momentum J is the spin, orbit (L + S) coupling If total L = L=0 L=1 L=2 L=3
(Ltotal = l1 + l2 + for each electron)
S symbol P symbol D symbol F symbol
Multiplicity: 2S + 1 is calculated from number of unpaired electrons S = 0 (all paired), S = 1/2 (one u.e.) , S = 1 (2 u.e.) , S = 3/2 (3 u.e.) , etc. Russell-Saunders Coupling: J ranges from L + S, , L-S and all integers in between
225
Ground-State Term Symbols for Any Electronic Configuration: The Ground State has: 1. Choose Maximum L value 2. Choose Maximum S value 3. Choose J value: (a) smallest J value if orbital sub-shell is less than half-filled (b) largest J value if orbital sub-shell is greater than half-filled
226
p2 Lmax = 1 P S=1 2S + 1 = 3 J = L+S .... L-S = 2, 1, 0 use lowest J value for < half-filled and highest J value for greater than half-filled
+1 3
-1
P0
3d3 [Ar] 3d 34s0 Lmax = 3 +2 4 +1 0 -1 -2 Smax = 3/2 F 2S + 1 = 4
F3/2
J = L+S .... L-S =9/2, 7/2, 5/2, 3/2 3/2 use lowest J value for < half-filled and highest J value for greater than half-filled
3d5 Mn2+ ion Lmax = 0 +2 6 +1 0 -1 -2 Smax = 5/2 S 2S + 1 = 6
J = L+S .... L-S =5/2
S5/2
227
Determining Energy States from Orbital Configurations
(after Russell-Saunders Term symbols now what?)
Strong-field Cases (very different than free ions) The ligand field dominates, therefore energy states are determined by the symmetries of the orbitals in which the electrons reside. FIVE cases in determining Electronic States and their spin multiplicities from Electronic Configurations (orbital designations)
Note: orbitals have lower case letters & states have capital letters
Case 1: All orbitals are fully occupied. (a) Multiplicity (2S + 1) with S = 0 (b) electron distribution is totally symmetric
Oh Cr(CO)6 t2g6eg0
1
C4v Co(NH3)5X]2+
A1g
b22e4aa10b10 b1 a1
A1
eg
t2g b2
228
Case 2: Non-degenerate orbital that is singlyoccupied. (a) Multiplicity (S = 1/2): (2S + 1) = 2 or doublet (b) if symmetry of the occupied orbital is X, then the State Symmetry is 2X.
C4v [Co(NH3)5X]+ b22e4aa11b10 b1 a1 e b2
2
A1
Case 3: two singly occupied, non-degenerate orbitals (a) Multiplicity two cases to consider S=0, paired electrons, 2S+1 = 1 or a singlet S=1, two unpaired electrons, 2S+1 = 3 or a triplet (b) State Symmetry is the direct product of the symmetry of the electrons in the two orbitals X1 X2 (these are the representations in the point groups)
229
[Pt(CN)4]2square planar D4h a1g2eg4b2g1b1g1 B2g B1g = A2g (in D4h p.g.) So you get two states:
3
Excited State for a d2 ion t2g1eg1 in Oh T2g x Eg = T2g + T1g
1
T2g, 3T2g, 1T1g, 3T1g
A2g, 1A2g
all spins possible
Question How do you multiply X1 X2, where X1 and X2 are representations in the point group? B2g B1g = ? Answer Multiply the characters (numbers) for the representations in D4h character table
230
Question What if inspection does not work? Then you must reduce the new representation to a sum of certain representations in the group.
you must use the reduction formula: ai = 1/h ( R iR n) ai refers gives you the number of a particular irred. rep. that is contained in the reducible representation 1/h (h is the order of the group = total # of operations)
R = character of the new (reducible representation) iR = character of the irreducible rep. in the group
231
For Oh h = order of group = 48 operations n = varies for each column
example:
Oh A1g E 1 8C3 1 6C2 6 C4 1 1
n = 8 for this class n = 6 for this class
T2g x Eg => reduce using the formula #A1g: 1/48[(6 x 1 x 1) + 0 + 0 + 0 + (-2 x 1 x 3) + (6 x 1 x 1) + 0 + 0 + (-2 x 1 x 3) + 0] [6 6 + 6 6] = 0 #A2g: #Eg: =>0 =>0 1/48[(6 x 3 x 1) + 0 + 0 + 0 + (-2 x 1 x 3) + (6 x 3 x 1) + 0 + 0 + (-2 x -1 x 3) + 0] : 1/48[18 + 0 + 0 + 0 + 6 + 18 + 6] = 1/48[48] = 1
#T1g:
#T2g:
1/48[(6 x 3 x 1) + 0 + 0 + 0 + (-2 x -1 x 3) + (6 x 3 x 1) + 0 + 0 + (-2 x -1 x 3) + 0] = 18 + 6 + 18 + 6 = 1 T2g x Eg = T1g + T2g
Therefore:
232
and since there are two electrons in different orbitals t2g1eg1 => it leads to both singlet and triplet states for both Energy Terms:
T1g 1 T2g
T1g 3 T2g
Case 4: Degenerate orbitals with a hole (lacking 1e- to be filled) (a) hole formalism a degenerate orbital with a hole is equivalent to same degenerate orbital occupied by a single e-. (b) Multiplicity S = 1/2 (2S + 1 = 2) doublet state
e.g. [Ru(bpy)3]3+ => t2g5eg0 2T2g [Mn(CN)6]4- => same
233
Case 5: Two electrons in a degenerate orbital (or nelectrons in a degenerate orbital)
a) state symmetry is given by X X (direct product of the orbital symmetry) b) *multiplicity can be a triplet or a singlet * Problem is matching the correct symmetry to multiplicity! i.e. it must obey the Pauli principle (if 2 electrons have the same spin, they must occupy different orbitals or energy states)
Example in Oh t2g6eg2 Multiply Eg x Eg = ?A1g + ?A2g + ?Eg (the irreducible reps. that arise from Eg x Eg) you must match the symmetry with the multiplicity by lowering the symmetry. use correlation tables
a1g eg KEY IS TO LOWER THE SYMETRY FROM Oh to D4h removes b1g degeneracy
234
You can write two electrons in four different ways in these two orbitals A, B, C1 and C2
a1g b1g B C1 a1g b1g C2
a1g b1g A
a1g b1g
singlet and triplet
METHOD OF DESCENDING SYMMETRY
235
A. In D4h, a1g2 must be a singlet because of the Pauli Principle (if two electrons are in the same orbital, they must have opposite spins). a1g2 is a case 1 situation so state symbol is 1A1g B. In D4h, b1g2 must be a singlet for same reason above. b1g2 is a case 1 situation so state symbol is again 1A1g
C. b1g1a1g1 (triplet)
b1g1a1g1 (singlet)
(both are case 3). Multiply B1g x A1g in D4h point group => reduces to B1g 1 B1g 3B1g Now go to correlation table (p. 437 Cottons Group Theory Book) and find the correspondence between Oh and D4h point groups.
236
Oh
?
?
D4h
___----------------------------------
A2g ? Eg
A1g
?
? ?
B1g A1g ? B1g
1
A1g
D4h 1 A1g
1
We wind up with A1g B1g B1g A1g
These are the three state multiplicities that we are trying to assign. Lowering the symmetry cannot change the multiplicity
1
Oh
1
Eg
B1g B1g A1g
3
3 1
A2g A1g
Example Two: Case 5: Assigning State Terms to Orbital Configurations where 2 electrons are in a degenerate set of orbitals. Oh t2g2 (T2g x T2g) => reducible representation
use mathematical formula to reduce it: you get =>
?
A1g + ?Eg +?T1g +?T2g
237
We must lower the symmetry far enough such that all E (two-dimensional and T (threedimensional) representations become onedimensional (or a sum of one-dimensional) representations. (look at Correlation Table)
We have chosen to go all the way down to C2h (preserves center of symmetry) Oh C2h A1g Eg T1g T2g Ag Ag + Bg Ag + Bg + Bg (Ag + 2Bg) Ag + Ag + Bg (2Ag + Bg)
Now go back to the orbitals:
There are six different ways that two e- can go into these orbitals in a C2h point group orbital diagram:
bg ag2 ag1 1 *2 bg ag2 ag1 *3 bg ag2 ag1 4 bg ag2 ag1 *5 bg ag2 ag1 6 bg ag2 ag1
*These have singlet versions as well as the triplets shown here
238
Now, we must determine the states corresponding to these orbital configurations. (all of these can be solved by inspection of character table) (1)* ag1 ag1 = Ag (2) ag1 ag2 = Ag (3) ag1 bg = Bg (4)* ag2 ag2 = Ag (5) ag1 bg = Bg (6)* bg bg = Ag (1)*, (4)*, (6)* must be singlets because the electrons are in the same orbital => 1Ag, 1Ag, 1Ag (2), (3), (5) can be both singlets and triplets => 1Ag, 3Ag 1Bg, 3Bg 1Bg, 3Bg to summarize (1) 1Ag (2) 1Ag 3Ag (3) 1Bg 3Bg (4) 1Ag (5) 1Bg 3Bg (6) 1Ag Remember, we had earlier determined that T2g x T2g = ?T2g + ?T1g + ?Eg + ?A1g and T2g = 2Ag + Bg T1g = Ag + 2Bg Eg = Ag + Bg A1g = Ag
239
Now make the correlation:
Oh
1
C2h
1 1
Make the connections from
Ag Eg T2 g
Ag
lower symmetry on the right hand side to her higher symmetry on the left. The correlations are unique- this is the only way that it would work. The two triplet 3Bg 's must go with
T1g because there is only one 3A g
Ag
1 1 1
Bg
Ag Bg
Ag
1 3 3
T1g
Bg Bg Ag
to go with them
240
Two extremes of considering significance of L-S or spin-orbit coupling: L-S coupling is the coupling of the magnetic dipole of electron spin moment with the orbital moment
1. Russell-Saunders in this scheme e-/e- repulsion dominates and spin-orbit coupling (L-S) coupling is treated as a perturbation on e-/e- repulsion. L-S coupling is small with first row T-metals (second row also). 2. j-j coupling in this scheme, the L-S coupling dominates and maximum J values are used to describe states and e-/e- repulsion is a perturbation on these states. Third row T-metals and rare earth elements must be treated in this way.
241
242
How do you get ordering of these? Consider e-/e- repulsions as being important (as before in free ions) How would you connect the Free ions to the strong-field cases? i.e. take Russell-Saunders Term symbols into orbital-based states without derivation Correlation of => Free ion RS States (L=0) (L=1) (L=2) (L=3) (L=4) S P D F G => => => => => Energy States of Orbital A1 T1 E + T2 A2 + T1 + T2 E + T1 + T2 + A1
Tanabe-Sugano Diagrams use lines to connect free ion states to molecular states.
Das könnte Ihnen auch gefallen
- Tables of Coefficients for the Analysis of Triple Angular Correlations of Gamma-Rays from Aligned NucleiVon EverandTables of Coefficients for the Analysis of Triple Angular Correlations of Gamma-Rays from Aligned NucleiNoch keine Bewertungen
- Ten-Decimal Tables of the Logarithms of Complex Numbers and for the Transformation from Cartesian to Polar Coordinates: Volume 33 in Mathematical Tables SeriesVon EverandTen-Decimal Tables of the Logarithms of Complex Numbers and for the Transformation from Cartesian to Polar Coordinates: Volume 33 in Mathematical Tables SeriesNoch keine Bewertungen
- Group Theory-Part 12 Correlation DiagramsDokument36 SeitenGroup Theory-Part 12 Correlation Diagramsnancyboy100% (1)
- SPECTRAL CALCULATION OF ORGEL AND TANABE-SUGANO DIAGRAMSDokument18 SeitenSPECTRAL CALCULATION OF ORGEL AND TANABE-SUGANO DIAGRAMSTiwari VishalNoch keine Bewertungen
- Molecular Term SymbolsDokument5 SeitenMolecular Term SymbolsAdam ButterworthNoch keine Bewertungen
- Molecular Term SymbolsDokument5 SeitenMolecular Term Symbols1jerushaNoch keine Bewertungen
- Electrochemistry 12Dokument19 SeitenElectrochemistry 12Manas ChhabraNoch keine Bewertungen
- Goldstein 2nd Edition 2nd Chapter SolutionsDokument10 SeitenGoldstein 2nd Edition 2nd Chapter SolutionsKarmakarışıkblog Blogspot67% (3)
- Chem 373 - Lecture 20: Complex Atomic SpectraDokument27 SeitenChem 373 - Lecture 20: Complex Atomic SpectraNuansak3Noch keine Bewertungen
- Molecular Orbital Theory of the H2 MoleculeDokument12 SeitenMolecular Orbital Theory of the H2 MoleculeSagarNoch keine Bewertungen
- Excitons in Bulk and Low-Dimensional SemiconductorsDokument9 SeitenExcitons in Bulk and Low-Dimensional Semiconductorsprakush_prakushNoch keine Bewertungen
- Tanabe-Sugano Diagrams and Ligand Field Splitting DeterminationDokument36 SeitenTanabe-Sugano Diagrams and Ligand Field Splitting DeterminationrakibNoch keine Bewertungen
- Lab Report - Tanabe-Sugano DiagramsDokument12 SeitenLab Report - Tanabe-Sugano DiagramsValerie MangasarNoch keine Bewertungen
- Electronic Spectra of ComplexesDokument82 SeitenElectronic Spectra of Complexesirembasar2000Noch keine Bewertungen
- Answers To Assignment2-2009Dokument6 SeitenAnswers To Assignment2-2009ElmIeyHakiemiEyNoch keine Bewertungen
- Spectra of ComplexesDokument41 SeitenSpectra of ComplexesSheena GagarinNoch keine Bewertungen
- CH 14. Electronic Spectros PDFDokument9 SeitenCH 14. Electronic Spectros PDFiq3pevicNoch keine Bewertungen
- Lecture 14111Dokument30 SeitenLecture 14111--Noch keine Bewertungen
- 2010 Fall With SolutionsDokument36 Seiten2010 Fall With SolutionsRay MondoNoch keine Bewertungen
- Term Symbols and Selection Rules in Atomic SpectroscopyDokument4 SeitenTerm Symbols and Selection Rules in Atomic SpectroscopyIqbal Aljabir PujionoNoch keine Bewertungen
- Chemistry 445 Lecture 18 MO Theory and D-D SpectraDokument22 SeitenChemistry 445 Lecture 18 MO Theory and D-D SpectraAbhinav JainNoch keine Bewertungen
- Past Papers Solutions OutputDokument35 SeitenPast Papers Solutions OutputCharlie Biopunk AlesNoch keine Bewertungen
- Ahn 1986Dokument16 SeitenAhn 1986achmad muzammilNoch keine Bewertungen
- 10 5Dokument15 Seiten10 5AZIZ ALBAR ROFI'UDDAROJADNoch keine Bewertungen
- Ch3 ExamplesDokument11 SeitenCh3 ExamplesMariana LizethNoch keine Bewertungen
- Degrees of Freedom X yDokument14 SeitenDegrees of Freedom X yDeviNoch keine Bewertungen
- A10 - Chap08A ExproDokument68 SeitenA10 - Chap08A Expro蔡易斈Noch keine Bewertungen
- Tanabe-Sugano Diagrams ExplainedDokument10 SeitenTanabe-Sugano Diagrams ExplainedAisha RehmanNoch keine Bewertungen
- Foot Atomic Solutions by Zhao, C.Dokument32 SeitenFoot Atomic Solutions by Zhao, C.Jacob Francis94% (16)
- Thanks To Yossef and Shiang Yong For Their Input in This ProblemDokument8 SeitenThanks To Yossef and Shiang Yong For Their Input in This ProblemIgnacio JuárezNoch keine Bewertungen
- Inorganic Spectoscopy-UV-VisDokument100 SeitenInorganic Spectoscopy-UV-VisUsama El-Ayaan100% (5)
- Adv Soln PDFDokument25 SeitenAdv Soln PDFSanchit MukherjeeNoch keine Bewertungen
- Solution Manual of Solid State Physics, Charles Kittles PDFDokument64 SeitenSolution Manual of Solid State Physics, Charles Kittles PDFYeo Jun Yi100% (1)
- CH2422 Electronic Spectra of Transition MetalsDokument6 SeitenCH2422 Electronic Spectra of Transition MetalsJohnNoch keine Bewertungen
- JSL-Lect 2 - Flame Emission and AAS - 16!10!21Dokument13 SeitenJSL-Lect 2 - Flame Emission and AAS - 16!10!21Divyansh SharmaNoch keine Bewertungen
- 6241 01 Rms 20050618Dokument10 Seiten6241 01 Rms 20050618UncleBulgariaNoch keine Bewertungen
- Lecture 5Dokument15 SeitenLecture 5NitinKumarNoch keine Bewertungen
- (Solutions) Kittel - Introduction To Solid State Physics 8Th EditionDokument60 Seiten(Solutions) Kittel - Introduction To Solid State Physics 8Th EditionCody Foster0% (1)
- Quantum Mechanics, Chapter 8Dokument7 SeitenQuantum Mechanics, Chapter 8oneoonineNoch keine Bewertungen
- Equilibri Quimic HW12 SolDokument8 SeitenEquilibri Quimic HW12 SolmarzinusNoch keine Bewertungen
- Angular Distribution Functions in The Decays of The D State of Charmonium Originating From Unpolarized PP CollisionsDokument8 SeitenAngular Distribution Functions in The Decays of The D State of Charmonium Originating From Unpolarized PP CollisionsEsther SimpsonNoch keine Bewertungen
- Solutions Tute 2Dokument9 SeitenSolutions Tute 2Felipe SibilaNoch keine Bewertungen
- Electronic Spectra of TM ComplexesDokument35 SeitenElectronic Spectra of TM Complexesbits_who_am_iNoch keine Bewertungen
- Iv 25% Xi CRP Che Iit Key 24-03-24Dokument5 SeitenIv 25% Xi CRP Che Iit Key 24-03-24pinnaacleclasses salemNoch keine Bewertungen
- Parte 3 TipeoDokument11 SeitenParte 3 TipeoMatamoros De La Cruz JorgeNoch keine Bewertungen
- Structural Theory DiscussionDokument8 SeitenStructural Theory Discussiontrishasdulay.enggNoch keine Bewertungen
- Jest QuestionDokument16 SeitenJest QuestionThirumugam SNoch keine Bewertungen
- SU (2) and Symmetry of The Dirac LagrangianDokument9 SeitenSU (2) and Symmetry of The Dirac LagrangianBhupendra BadgaiyanNoch keine Bewertungen
- Double PendulumDokument8 SeitenDouble PendulumLalu Sahrul HudhaNoch keine Bewertungen
- Time-ind. Perturbation TheoryDokument9 SeitenTime-ind. Perturbation Theorycuongspvl2713Noch keine Bewertungen
- Mathematics 1St First Order Linear Differential Equations 2Nd Second Order Linear Differential Equations Laplace Fourier Bessel MathematicsVon EverandMathematics 1St First Order Linear Differential Equations 2Nd Second Order Linear Differential Equations Laplace Fourier Bessel MathematicsNoch keine Bewertungen
- Practice Makes Perfect in Chemistry: Oxidation-ReductionVon EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionBewertung: 5 von 5 Sternen5/5 (1)
- Advanced Electric Circuits: The Commonwealth and International Library: Applied Electricity and Electronics DivisionVon EverandAdvanced Electric Circuits: The Commonwealth and International Library: Applied Electricity and Electronics DivisionNoch keine Bewertungen
- A Course of Mathematics for Engineers and ScientistsVon EverandA Course of Mathematics for Engineers and ScientistsNoch keine Bewertungen
- Electron Beam-Specimen Interactions and Simulation Methods in MicroscopyVon EverandElectron Beam-Specimen Interactions and Simulation Methods in MicroscopyNoch keine Bewertungen
- Power Theft Identification Using GSM TechnologyDokument3 SeitenPower Theft Identification Using GSM TechnologyIJORAT100% (1)
- Admix Load CellDokument6 SeitenAdmix Load Cellmanil_5Noch keine Bewertungen
- 2N3866 Datasheet Rev BDokument5 Seiten2N3866 Datasheet Rev Bardier34Noch keine Bewertungen
- Crystal Chem Crystallography: - Chemistry Behind Minerals and How They Are AssembledDokument33 SeitenCrystal Chem Crystallography: - Chemistry Behind Minerals and How They Are AssembledArkodip MandalNoch keine Bewertungen
- THKDokument1.901 SeitenTHKapi-26356646Noch keine Bewertungen
- LTE and SchedulingDokument25 SeitenLTE and SchedulingKrunoslav IvesicNoch keine Bewertungen
- Introduction - Week 2Dokument37 SeitenIntroduction - Week 2Tayyab AhmedNoch keine Bewertungen
- Tips and Tricks I: Getting the Most Out of ArcGIS DesktopDokument108 SeitenTips and Tricks I: Getting the Most Out of ArcGIS Desktoptanja222Noch keine Bewertungen
- Facilities Assignment 1-2-2015Dokument2 SeitenFacilities Assignment 1-2-2015Xnort G. Xwest0% (1)
- Stereochemistry Practce PDFDokument6 SeitenStereochemistry Practce PDFFerminNoch keine Bewertungen
- Solutions To Irodov's Problems - Volume IIDokument442 SeitenSolutions To Irodov's Problems - Volume IIZequinha de Abreu100% (2)
- Shares Dan Yang Belum Diterbitkan Disebut Unissued SharesDokument5 SeitenShares Dan Yang Belum Diterbitkan Disebut Unissued Sharesstefanus budiNoch keine Bewertungen
- Huawei Mediapad m5 10.8inch Ръководство За Потребителя (Cmr-Al09, 01, Neu)Dokument6 SeitenHuawei Mediapad m5 10.8inch Ръководство За Потребителя (Cmr-Al09, 01, Neu)Галина ЦеноваNoch keine Bewertungen
- Measurements/ Specifications: Torque Wrench Selection GuideDokument5 SeitenMeasurements/ Specifications: Torque Wrench Selection GuideSylvester RakgateNoch keine Bewertungen
- Excel Dynamic Arrays: Department Item Quantity Price Total $Dokument5 SeitenExcel Dynamic Arrays: Department Item Quantity Price Total $Bilal Hussein SousNoch keine Bewertungen
- Spesifikasi Produk SL-500Dokument2 SeitenSpesifikasi Produk SL-500tekmed koesnadiNoch keine Bewertungen
- Exponential & Logarithmic FunctionsDokument13 SeitenExponential & Logarithmic FunctionsRahul SankaranNoch keine Bewertungen
- Visualizing Interstellar WormholesDokument14 SeitenVisualizing Interstellar WormholesFranciscoNoch keine Bewertungen
- SubNetting Practice LabDokument3 SeitenSubNetting Practice LabOdoch HerbertNoch keine Bewertungen
- Valve Actuator Data SheetDokument1 SeiteValve Actuator Data SheetRob StorrowNoch keine Bewertungen
- Steps For Charm ConfigurationDokument7 SeitenSteps For Charm ConfigurationpoornasapNoch keine Bewertungen
- DasibiOzoneMonitorManual 1008Dokument183 SeitenDasibiOzoneMonitorManual 1008api-26966403100% (2)
- Introducing WESAD, A Multimodal Dataset For Wearable Stress and Affect DetectionDokument9 SeitenIntroducing WESAD, A Multimodal Dataset For Wearable Stress and Affect DetectionJhónatan CarranzaNoch keine Bewertungen
- ASTM D 1510 - 02 Carbon Black-Iodine Adsorption NumberDokument7 SeitenASTM D 1510 - 02 Carbon Black-Iodine Adsorption Numberalin2005100% (1)
- Impedance Measurement Handbook: 1st EditionDokument36 SeitenImpedance Measurement Handbook: 1st EditionAlex IslasNoch keine Bewertungen
- Max Born, Albert Einstein-The Born-Einstein Letters-Macmillan (1971)Dokument132 SeitenMax Born, Albert Einstein-The Born-Einstein Letters-Macmillan (1971)Brian O'SullivanNoch keine Bewertungen
- Training Matrix For TM IDokument14 SeitenTraining Matrix For TM IApril NavaretteNoch keine Bewertungen
- Hawking-Brief History of TimeDokument336 SeitenHawking-Brief History of TimeAlbert Kristian0% (1)
- Design Plan: A Performance Task in GeometryDokument12 SeitenDesign Plan: A Performance Task in GeometryRobert Ryan SantiagoNoch keine Bewertungen
- 01-Jul-21 03:32 PMDokument6 Seiten01-Jul-21 03:32 PMramNoch keine Bewertungen