Beruflich Dokumente
Kultur Dokumente
Ion Suppression in LC-MS-MS - A Case Study
Hochgeladen von
msr_roniOriginalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Ion Suppression in LC-MS-MS - A Case Study
Hochgeladen von
msr_roniCopyright:
Verfügbare Formate
LC TROUBLESHOOTING
Ion Suppression in LCMSMS A Case Study
Michael D. Nelson, LC Resources, McMinnville, Oregon, USA and John W. Dolan, LC Resources, Walnut Creek, California, USA.
Sometimes a small change is all it takes.
Ion suppression in liquid chromatography coupled with mass spectrometry (LCMS) can occur when a coeluted compound suppresses the ionization of the sample molecules in a mass spectrometers source. This ion suppression is analogous to the large garbage peak for unretained material common at the beginning of chromatograms monitored by UV detection. Ion suppression, like other chromatographic interferences, compromises quantitative analysis because it can vary from sample to sample. Unfortunately, a tandem mass spectrometer operating in the multiple-reaction monitoring mode is just as susceptible as a single-stage unit. This months LC Troubleshooting examines a case in which ion suppression compromised method performance and shows how the strategy for eliminating ion suppression differs little from addressing other chromatographic interferences.
problems. First, infuse a dilute solution of the analyte at a constant rate into the effluent flowing from the LC system to the mass spectrometer to create an elevated but constant baseline. After obtaining a steady baseline, inject a blank sample extract into the LC system. Any eluted material that suppresses ionization in the mass spectrometer will cause a drop in the baseline. Figure 2(a) shows a drop in the region between 0.7 and 1.0 min. Ion suppression can sometimes be eliminated by improved sample preparation, but generally it is easier to adjust the chromatographic conditions so the peaks of interest are not eluted in the suppression region, as was the situation for the paclitaxel sample in Figure 2(b).
The Current Problem
Our laboratory had developed an LCMSMS method to analyse a proprietary pharmaceutical compound and one of its metabolites. The method appeared to be ready for validation until
Identifying Ion Suppression
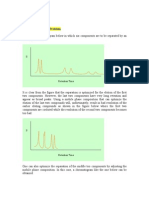
Figure 1 illustrates the standard way to screen for potential ion suppression
we moved it from one mass spectrometer to another of the same brand and model. At this point, the method performance deteriorated dramatically, especially with lower concentrations of analytes extracted from plasma. Urine extracted in the same manner acted normally. Unfortunately, equipment availability prevented us from moving back to the original LCMSMS system. We suspected ion suppression was the problem source, so we performed the infusion experiment described above with the results shown in Figure 3. The lower trace shows the infusion experiment overlaid on the upper chromatogram of a standard of the three sample components of interest. The figure shows that the parent drug is eluted at the beginning of a large suppression region. Small changes in retention or variations in the sample matrix could easily change the relative contribution of the suppression and analyte signals and reduce the method performance. We had confirmed that ion suppression was a likely cause of our problem.
Figure 1: Experimental set-up for determining ion suppression with LCMSMS.
See text for details
Chromatographic Changes
Although considerably broader than normal chromatographic peaks, ion suppression regions can be moved in the same manner as normal peaks by manipulating chromatographic conditions. We tried several variations in the separation conditions in an attempt to correct the problem. For gradient elution, a change in gradient slope is analogous to a change in mobile-phase strength in isocratic separations; therefore, it is a powerful way to change chromatographic selectivity. The
LCGC Europe February 2002
LC Troubleshooting
A change in the mobile phases organic solvent content can provide dramatic selectivity changes in LC separations.
initial separation (Figure 3) used a 6.25%/min gradient starting at 5% acetonitrile. Changing to a steeper gradient of 11.25%/min moved the main peak further into the suppression region (Figure 4(a)). A shallower gradient (Figure 4(b)) caused a similar change. Clearly, gradient steepness alone was an insufficiently powerful tool to solve the current problem. A change in the mobile phases organic solvent content can provide dramatic selectivity changes in LC separations, so we thought switching from acetonitrile
Figure 2: Chromatograms showing retention times for (a) the ion suppression region
and (b) the analytes of interest in a method for measuring paclitaxel in plasma.
Figure 3: Chromatograms obtained using the conditions under which the ion
suppression problem was originally discovered. The ion suppression trace is shown on the bottom. Column: 75 mm 4.6 mm ODS-3; mobile-phase A: 0.05% heptafluorobutyric acid in water; mobile-phase B: 0.05% heptafluorobutyric acid in acetonitrile; gradient: 530% B in 4 min; dwell volume: 2.2 mL.
Peaks: 1
metabolite, 2
internal standard, 3
parent drug.
to methanol would be a logical choice to explore this variable. This change moved the main peak to the back edge of the suppression region (Figure 4(c)), but it was also insufficient to avoid ion suppression. Heptafluorobutyric acid at a concentration of 0.05% was present in the mobile phase to control the pH and to act as an ion-pairing reagent. We decided to explore the effects of a change in heptafluorobutyric acid concentration. Increasing the heptafluorobutyric acid concentration to 0.1% resulted in all the peaks being eluted in less than 1.5 min and no retention for the metabolite; clearly, it was an undesirable effect. Reducing the heptafluorobutyric acid concentration to 0.025% changed selectivity (Figure 4(d)), but the main peak still overlapped with the suppression region. As when developing a new chromatographic method, the next logical step after modifying mobile-phase strength (gradient steepness) and mobile-phase chemistry is to look at the effect of changing the stationary phase. We performed the original separation on a Type B silica C18 column (Inertsil ODS-3, Metachem, Torrance, California, USA). It is best to try a dramatic change in stationary phase for a change in selectivity, so we tried an embedded polar phase (Symmetry Shield, Waters Corp., Milford, Massachusetts, USA) but were unable to find conditions that would give sufficient retention for the polar metabolite. Although changing from one manufacturers C18 column to anothers is generally a poor way to change selectivity, we were beginning to run out of options. A sterically protected C18 column (StableBond C18, Agilent Technologies, Wilmington, Delaware, USA) changed the profile of the suppression region (Figure 4(e)) but did not provide enough selectivity change. A low-silica C18 column (XTerra, Waters) provided the best separation so far (Figure 4(f)). It was very close to the separation we had observed before changing instruments (Figure 5), but it still didnt leave much margin for error. Note that the XTerra column was 150 mm long, whereas the other columns were 75 mm long. For this reason, the gradient time was increased to 8 min to keep the gradient time and flow-rate constant and to obtain the same effective gradient steepness. We attribute the change in selectivity to the change in column chemistry and not the change in gradient conditions because of this change.
3
LCGC Europe February 2002
LC Troubleshooting
Whats Left?
After carefully reviewing the data and efforts to change selectivity, we realized that we had failed to examine the effect of system
dwell volume on the separation. The dwell volume is the system volume from the point where the solvents are mixed to the point where they reach the inlet of the column.
The most common effect of dwell-volume changes is a shift in retention for all peaks accompanied by small changes in selectivity. Large changes in selectivity are less common.
Figure 4: Modification of conditions of Figure 3. All conditions were as in Figure 3 except as noted. Shown are results obtained using (a) a steeper gradient, (b) a shallower gradient, (c) methanol instead of acetonitrile as B solvent, (d) 0.025% instead of 0.05% heptafluorobutyric acid, (e) an SB-C18 column and (f) a 150 mm 4.6 mm XTerra column.
LCGC Europe February 2002
LC Troubleshooting
We examined the system records and determined that the original separation (Figure 5) was performed on an LC system using low-pressure mixing (one pump) with a measured dwell volume of 0.88 mL. The remaining chromatograms shown above were obtained with a high-pressure mixing system (two pumps) with a dwell volume of 2.2 mL. Comparing the chromatograms for these two systems (Figures 3 and 5) suggested that a smaller dwell volume moved the main peak forward relative to the suppression region. We previously had used a high-pressure mixing system with this method, and it provided satisfactory results, so the change was a bit surprising. Further digging in the system records, however, showed that the earlier work with the high-pressure mixing system had used a micromixer instead of the standard mixer. We normally use the micro-mixer for LCMSMS work for small columns operated at low flow-rates for example, 50 2.1 mm columns operated at 0.2 mL/min but the micromixer is less advantageous for conventional 75 4.6 mm or 150 4.6 mm columns operated at higher flow-rates (1.5 mL/min, in this instance). It appeared that this assumption was wrong for the present case. To test our hypothesis, we replaced the conventional mixer with a micromixer (0.5 mL) and observed the results shown in Figure 6. Obviously, dwell volume was the key parameter in this situation. The reduced dwell volume had the same effect with the original column (not shown).
The most common effect of dwell-volume changes is a shift in retention for all peaks accompanied by small changes in selectivity.
isocratic elution) first, because it was easy and fairly powerful. Next, we changed the mobile-phase chemistry because chemistry changes are more likely to affect selectivity than are column changes. When we changed columns, it was first to a different type of column (embedded polar phase), which was more likely to change selectivity than a change from one manufacturers C18 to another.
Figure 5: Results obtained using the same conditions as in Figure 3 but with a 0.88 mL dwell volume.
Figure 6: Results obtained using the same conditions as in Figure 4(f) but with a micromixer (0.5 mL dwell volume).
Conclusions
What can we learn from this exercise? When we first discovered the method performance problem, it manifested itself primarily in a fall-off in precision and accuracy at lower analyte concentrations. We were distracted by a suspicion that the freshly prepared standards were diluted improperly. By the time we discovered that the problem was related to ion suppression, we failed to recognize the instrument change as a factor. We approached the problem in a logical, stepwise manner: We changed the separation variables using a tried-and-true procedure that took advantage of a combination of the ease of making a change and the leverage, or probability, that the variable would cause a change in selectivity. So, we changed the gradient steepness (equivalent to solvent strength in
LCGC Europe February 2002
LC Troubleshooting
Eliminating ion suppression problems is no different than dealing with other chromatographic interferences.
That the problem was finally traced to a dwell-volume change makes us a bit redfaced we should have noticed it at the beginning. On the positive side, the exercise produced a nice example of the approach to addressing an ion suppression problem. Eliminating ion suppression problems is no different than dealing with other chromatographic interferences either eliminate the suppression peak through sample clean-up or modify the chromatographic conditions so it does not cause a problem.
Michael Nelson is a chemist at LC Resources Inc., McMinnville, Oregon, USA. He specializes in LCMSMS determination of pharmaceuticals in biological matrices. LC Troubleshooting editor John W. Dolan is president of LC Resources Inc., of Walnut Creek, California, USA, and a member of the Editorial Advisory Board of LCGC Europe. Direct correspondence about this column to LC Troubleshooting, LCGC Europe, Advanstar House, Park West, Sealand Road, Chester CH1 4RN, UK. Readers can also direct questions to the on-line Chromatography Forum at http://www.chromforum.com
NEXT MONTH in LC Troubleshooting... Quiet Please How can you reduce baseline noise?
TELL US WHAT YOU THINK...
If you have any comments on this article please e-mail them to: dhills@advanstar.com
LCGC Europe February 2002
Das könnte Ihnen auch gefallen
- Amino Acids, Peptides and Proteins in Organic Chemistry 2 - Modified Amino Acids, Organocatalysis and Enzymes (Amino Acids, Peptides and Proteins in Organic Chemistry (VCH) ) (PDFDrive)Dokument695 SeitenAmino Acids, Peptides and Proteins in Organic Chemistry 2 - Modified Amino Acids, Organocatalysis and Enzymes (Amino Acids, Peptides and Proteins in Organic Chemistry (VCH) ) (PDFDrive)John Carlo QuiatchonNoch keine Bewertungen
- Agilent Pesticides App CompendDokument753 SeitenAgilent Pesticides App CompendKiran Chokshi100% (1)
- HPLC 08Dokument13 SeitenHPLC 08Vikas SharmaNoch keine Bewertungen
- Multidimention Why Need More ResearchDokument14 SeitenMultidimention Why Need More Researchkassim AliNoch keine Bewertungen
- Artigo Journal Chemical EducationDokument4 SeitenArtigo Journal Chemical EducationMaíra PradoNoch keine Bewertungen
- A Comparison of CE-MS and LC-MS For Peptide SamplesDokument6 SeitenA Comparison of CE-MS and LC-MS For Peptide SamplesPienovNoch keine Bewertungen
- Lle Design PaperDokument14 SeitenLle Design PaperRamesh ReddyNoch keine Bewertungen
- LCMS Introduction Part 2Dokument34 SeitenLCMS Introduction Part 2rostaminasabNoch keine Bewertungen
- GlycerolDokument10 SeitenGlycerolAshwani KumarNoch keine Bewertungen
- Anal Chem 84 (2012) 1474-1482Dokument9 SeitenAnal Chem 84 (2012) 1474-1482Verónica VanderhoevenNoch keine Bewertungen
- CHM510-e - LaboratoryManual Sem October 2020-Februari2021Dokument10 SeitenCHM510-e - LaboratoryManual Sem October 2020-Februari2021Amirah AzlanNoch keine Bewertungen
- Novel Approach to Job's MethodDokument6 SeitenNovel Approach to Job's Methodiabureid7460Noch keine Bewertungen
- Spectrophotometric Determination of The Stoichiometry of A ComplexDokument6 SeitenSpectrophotometric Determination of The Stoichiometry of A ComplexDean Dela CruzNoch keine Bewertungen
- chm510 Exp2Dokument10 Seitenchm510 Exp2May LeeNoch keine Bewertungen
- Elphor Chrom AnswersDokument3 SeitenElphor Chrom AnswersAnonymous b14ATJtNoch keine Bewertungen
- Recommendations to Improve Accuracy of Saponification Reaction Experiment in a Plug Flow ReactorDokument2 SeitenRecommendations to Improve Accuracy of Saponification Reaction Experiment in a Plug Flow ReactorHazirah AjamNoch keine Bewertungen
- HPLC 137.1Dokument9 SeitenHPLC 137.1Rehana LebbeNoch keine Bewertungen
- Lipase Enzyme Assay Final FinalDokument4 SeitenLipase Enzyme Assay Final FinalFlóra DomjánNoch keine Bewertungen
- How Much Retention Time Variation is NormalDokument10 SeitenHow Much Retention Time Variation is NormalDiego PivatoNoch keine Bewertungen
- Improved Extraction of THC and Its Metabolites From Oral Fluid Using Oasis Prime HLB Solid Phase Extraction (Spe) and A Uplc Cortecs C18 ColumnDokument14 SeitenImproved Extraction of THC and Its Metabolites From Oral Fluid Using Oasis Prime HLB Solid Phase Extraction (Spe) and A Uplc Cortecs C18 ColumnSalinee KhamsaengNoch keine Bewertungen
- Enzyme Liver Catalase Hydrogen PeroxideDokument9 SeitenEnzyme Liver Catalase Hydrogen PeroxideCarmen Muir100% (2)
- HPLC Performance Verification GuideDokument6 SeitenHPLC Performance Verification GuideMarwa Abdel RahmanNoch keine Bewertungen
- Enzyme CatalysisDokument15 SeitenEnzyme CatalysisMaelyn Nicole RominNoch keine Bewertungen
- HPLC Analysis of AspirinDokument4 SeitenHPLC Analysis of AspirinRazel Elaine Grace CataluñaNoch keine Bewertungen
- The General Elution ProblemDokument3 SeitenThe General Elution ProblemgoodigoodiNoch keine Bewertungen
- Lecture 7 (MT Resistances in Immobilized Enzyme)Dokument21 SeitenLecture 7 (MT Resistances in Immobilized Enzyme)sanyukta sinha100% (1)
- CKB 20104 Reaction Engineering UniKL MICET Experiment 3a: Effect of Residence Time On The Reaction in A PFR Full Lab ReportDokument20 SeitenCKB 20104 Reaction Engineering UniKL MICET Experiment 3a: Effect of Residence Time On The Reaction in A PFR Full Lab ReportSiti Hajar MohamedNoch keine Bewertungen
- BME 103 Homework 4Dokument4 SeitenBME 103 Homework 4Martin LuNoch keine Bewertungen
- ElectromembraneExtractionLCGC Feb2010Dokument15 SeitenElectromembraneExtractionLCGC Feb2010Lisette BecerraNoch keine Bewertungen
- 322 Fall 2014 Exam 2 KeyDokument7 Seiten322 Fall 2014 Exam 2 KeyAlexaNoch keine Bewertungen
- 148999T 2CEM20 AT1 DesignPracticalDJDokument34 Seiten148999T 2CEM20 AT1 DesignPracticalDJJoey YanNoch keine Bewertungen
- Proteins and Amino Acids PreDokument6 SeitenProteins and Amino Acids PreKarina KhanNoch keine Bewertungen
- Conducivity PH NotesDokument11 SeitenConducivity PH NotesRakesh RanjanNoch keine Bewertungen
- Emulsion Polymerization of Styrene with iOMPDokument9 SeitenEmulsion Polymerization of Styrene with iOMPGilar GumelarNoch keine Bewertungen
- Informe Osmosis InversaDokument7 SeitenInforme Osmosis InversaLina BeltranNoch keine Bewertungen
- Improving LC-MS Sensitivity Through Increases in Chromatographic Performance: Comparisons of UPLC-ES/MS/MS To Hplc-Es/Ms/MsDokument10 SeitenImproving LC-MS Sensitivity Through Increases in Chromatographic Performance: Comparisons of UPLC-ES/MS/MS To Hplc-Es/Ms/MsgannysunNoch keine Bewertungen
- DydasDokument9 SeitenDydasKatrina RosalbaNoch keine Bewertungen
- Tittle: High Performance Liquid Chromatography (HPLC) Method DevelopmentDokument7 SeitenTittle: High Performance Liquid Chromatography (HPLC) Method DevelopmentSiti FalaeinNoch keine Bewertungen
- Gradient Performance ChecksDokument3 SeitenGradient Performance ChecksKthePovedaNoch keine Bewertungen
- Experiment 2 Enzyme Assays and Factors Affectingenzyme ActivityDokument15 SeitenExperiment 2 Enzyme Assays and Factors Affectingenzyme Activitymohamad ashaziq100% (4)
- AN63754 - E 10-13S Opiates & Benzos - St7-HIGHDokument8 SeitenAN63754 - E 10-13S Opiates & Benzos - St7-HIGHmikicacicaNoch keine Bewertungen
- Compounds Are Operated at This Range: 3.full Name of OOS & OOT ? Dercribe It With Details ? Why This Is Needed inDokument10 SeitenCompounds Are Operated at This Range: 3.full Name of OOS & OOT ? Dercribe It With Details ? Why This Is Needed inRohit SharmaNoch keine Bewertungen
- Discussion Conclusion RecommendationDokument2 SeitenDiscussion Conclusion RecommendationFaiz BasriNoch keine Bewertungen
- HPLC of Methyl Salicylate in A Medicated CreamDokument10 SeitenHPLC of Methyl Salicylate in A Medicated CreamJuan PerezNoch keine Bewertungen
- Practice Problem Set 12 HPLCDokument12 SeitenPractice Problem Set 12 HPLCYocobSamandrewsNoch keine Bewertungen
- Laboratory measurements of liquid permeability discrepanciesDokument12 SeitenLaboratory measurements of liquid permeability discrepanciesRosa K Chang HNoch keine Bewertungen
- Hyphemated TechniquesDokument14 SeitenHyphemated TechniquesVijay PradhanNoch keine Bewertungen
- Derivatization Methods in GC and GCDokument32 SeitenDerivatization Methods in GC and GCLorena De La CruzNoch keine Bewertungen
- Adsorption of Methyl Tert-Butyl Ether Using Granular Activated Carbon: Equilibrium and Kinetic AnalysisDokument8 SeitenAdsorption of Methyl Tert-Butyl Ether Using Granular Activated Carbon: Equilibrium and Kinetic Analysisrarunr1Noch keine Bewertungen
- Comparison Relation To Retention Time Relative Retention ResolutionDokument13 SeitenComparison Relation To Retention Time Relative Retention Resolutionandreas hpNoch keine Bewertungen
- Experiment 8 FinalDokument14 SeitenExperiment 8 Finalemjd1100% (1)
- Open Chemistry JournalDokument16 SeitenOpen Chemistry JournalbhavanaNoch keine Bewertungen
- Physical Pharmacy I-II Sem JNTUK Lab ManualDokument75 SeitenPhysical Pharmacy I-II Sem JNTUK Lab ManualBabu Vij100% (4)
- Direct Methanol Fuel CellDokument6 SeitenDirect Methanol Fuel CellMarilynYunLingNoch keine Bewertungen
- ChemistryDokument4 SeitenChemistrypoyuerdeneNoch keine Bewertungen
- Unifac 6Dokument5 SeitenUnifac 6lester33Noch keine Bewertungen
- Standart SolutionDokument17 SeitenStandart SolutionC. A. ArdhaniNoch keine Bewertungen
- Analytical Characterization of BiotherapeuticsVon EverandAnalytical Characterization of BiotherapeuticsJennie R. LillNoch keine Bewertungen
- Computational Pharmaceutics: Application of Molecular Modeling in Drug DeliveryVon EverandComputational Pharmaceutics: Application of Molecular Modeling in Drug DeliveryDefang OuyangNoch keine Bewertungen
- Uv Vis Nir SpectrosDokument22 SeitenUv Vis Nir Spectrosmsr_roni100% (1)
- SOP For LabsolutionDokument6 SeitenSOP For Labsolutionmsr_roni83% (6)
- Col CareDokument5 SeitenCol Care829255Noch keine Bewertungen
- Buffers and BaselinesDokument3 SeitenBuffers and Baselinesmsr_roniNoch keine Bewertungen
- NDIR DetectorDokument1 SeiteNDIR Detectormsr_roniNoch keine Bewertungen
- Peak Purity Peak IdentificationDokument3 SeitenPeak Purity Peak Identificationmsr_roniNoch keine Bewertungen
- 16 Nexera DatasheetDokument1 Seite16 Nexera Datasheetmsr_roniNoch keine Bewertungen
- CO-PO MappingDokument6 SeitenCO-PO MappingArun Kumar100% (1)
- VacuumDokument7 SeitenVacuumMassimilianø Erricø100% (1)
- I-V Characterization of Tunnel Diodes and Multojunction Solar CellsDokument7 SeitenI-V Characterization of Tunnel Diodes and Multojunction Solar CellsMaura MusioNoch keine Bewertungen
- Pharmacology Review - A Comprehensive Reference Guide For Medical, Nursing, and Paramedic StudentsDokument276 SeitenPharmacology Review - A Comprehensive Reference Guide For Medical, Nursing, and Paramedic StudentsfjletonaNoch keine Bewertungen
- Lenox Catalogue PDFDokument40 SeitenLenox Catalogue PDFInvotexNoch keine Bewertungen
- ICSE Biology Exam 2021Dokument7 SeitenICSE Biology Exam 2021Sarthac JainNoch keine Bewertungen
- Silva Et Al., 2013 Coffee FerDokument13 SeitenSilva Et Al., 2013 Coffee FerYon SadisticNoch keine Bewertungen
- Carbohydrate Polymers: A B A B A B ADokument10 SeitenCarbohydrate Polymers: A B A B A B AKeiidys MartinezNoch keine Bewertungen
- 1D Nano Porous Silicon Optical Sensor Detects Methyl ParathionDokument7 Seiten1D Nano Porous Silicon Optical Sensor Detects Methyl ParathionhesoyamyecgaaaNoch keine Bewertungen
- Small Scale SoapmakingDokument82 SeitenSmall Scale SoapmakingDemelash GebreNoch keine Bewertungen
- DHEA CAPUYAN - Charles's Law Science ExperimentDokument4 SeitenDHEA CAPUYAN - Charles's Law Science ExperimentDhea Angela A. CapuyanNoch keine Bewertungen
- Ferrous and Non Ferrous Materials - Dr. ChalimbaDokument61 SeitenFerrous and Non Ferrous Materials - Dr. ChalimbaTadala Angella GomondaNoch keine Bewertungen
- CE2155 - 01 Mechanic of Materials (Part 3)Dokument18 SeitenCE2155 - 01 Mechanic of Materials (Part 3)Julia100% (1)
- Astm A500Dokument2 SeitenAstm A500Liquor Liam0% (1)
- 3.2.P.2.3 Manufacturing Process Development (92 Págs) PDFDokument92 Seiten3.2.P.2.3 Manufacturing Process Development (92 Págs) PDFaldoNoch keine Bewertungen
- Swimming Pool BOQ - Water ProofingDokument2 SeitenSwimming Pool BOQ - Water ProofingStephen RajNoch keine Bewertungen
- Sa 789 PDFDokument8 SeitenSa 789 PDFsergioprybyszNoch keine Bewertungen
- Eutronic - Arc - Spray 4HFDokument4 SeitenEutronic - Arc - Spray 4HFMuhammad irfanNoch keine Bewertungen
- Small STNTechnical ManualDokument8 SeitenSmall STNTechnical ManualMajid KhanNoch keine Bewertungen
- M.E.Forge Tech: Customer:M/s L & T Valves LimitedDokument1 SeiteM.E.Forge Tech: Customer:M/s L & T Valves LimitedK.s. Raghavendra KumarNoch keine Bewertungen
- Listening ComprehensionDokument3 SeitenListening ComprehensionLiz GilmoreNoch keine Bewertungen
- Kanthal LT CatalogDokument3 SeitenKanthal LT CatalogRichard SyNoch keine Bewertungen
- Innovative Use of Recycled Tyres in Civil Engineering ApplicDokument73 SeitenInnovative Use of Recycled Tyres in Civil Engineering Applicparasgandhi187874100% (5)
- T04: Mass Balance in Non Reacting System (Introduction Tu Multi Unit) A. Sugar Factory Activity (6 Min)Dokument2 SeitenT04: Mass Balance in Non Reacting System (Introduction Tu Multi Unit) A. Sugar Factory Activity (6 Min)Dewi Mawaddatus SholekhahNoch keine Bewertungen
- Pamphlet 001 Chlorine BasicsDokument61 SeitenPamphlet 001 Chlorine Basicsthorem100% (1)
- Cracking and Repair of Closing Welds in 2.25 Cr1 Mo Steel Vessels Operating in High Temperature Synthesis GasDokument9 SeitenCracking and Repair of Closing Welds in 2.25 Cr1 Mo Steel Vessels Operating in High Temperature Synthesis Gasvaratharajan g rNoch keine Bewertungen
- Cutting Tools TypeDokument3 SeitenCutting Tools TypeneurraNoch keine Bewertungen
- ALM Refrigeration Air DryerDokument34 SeitenALM Refrigeration Air DryerJunaid AhmedNoch keine Bewertungen
- Physico-chemical Water Treatment Processes AssignmentDokument6 SeitenPhysico-chemical Water Treatment Processes AssignmentAbir HasanNoch keine Bewertungen
- Overall Heat Transfer Coefficient and Pipe Length CalculationDokument2 SeitenOverall Heat Transfer Coefficient and Pipe Length CalculationCaleb FalcoteloNoch keine Bewertungen