Beruflich Dokumente
Kultur Dokumente
Discussion
Hochgeladen von
Azzian AriffinOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Discussion
Hochgeladen von
Azzian AriffinCopyright:
Verfügbare Formate
DISCUSSION In the experiment of determining of amount of Chromium (VI) water concentration in sample by using spectrophotometer and also to identify
whether the water sample is safe for drinking or at least, agriculture purpose. Chromium (VI) is used in this experiment as it is most found in rivers, lakes and streams. So to determine either Chromium (VI) is harmful or not, 5 series of diluted solution are prepared as the water sample and a spectrophotometer is used.
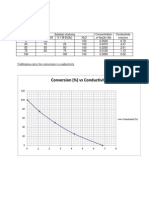
The spectrophotometer is an instrument which measures the amount of light of a specified wavelength which passes through a medium. According to Beer's law, the amount of light absorbed by a medium is proportional to the concentration of the absorbing material or solute present. The wavelength is set to 435nm. The concentration of Chromium (VI) in the lake water sample tested is 30.96 parts per million. The experiment is completed and successfully conducted. This experiment consist of two parts which is the first part is to determine the absorbance value of Chromium (VI) by using the (spectrophotometer. Before experiment, we need to do some calculation first to determine the volume of Chromium (VI) for the 5 different dilute solution. The equations of M1V1 = M2V2 is applied in order to calculate the volume of Chromium (VI). For the first dilute solution, the volume of Chromium (VI) used is 25mL, next is 12.5, 7.5, and 2.5mL. To dilute the solution, distilled water is added until the volume reached 50mL, thats mean each solution have the different dilution. For concentration of Chromium (VI) in parts per million (p.p.m), we are using 5 different value which is 150 ppm, 75, 45, and 15 ppm. Hence the absorbance value for each dilution is 0.377, 0.175, 0.110, 0.018. For the second part which is the determination of Chromium (VI) in sample of lake water. The absorbance value is 0.136 an after calculation, the concentration of Chromium (VI) in lake water is 58.8 ppm.
The graph of absorbance value against concentration of Chromium (VI) in parts per million (p.p.m) is directly proportional. According to the theory, we should get the best line fit graph, so our graph can be considered correct. Based on the graph, we need to calculate the volume of Chromium (VI) used when we are using sample of lake water by using the gradient. The equation of the line is y = 0.0026x 0.0171. In order to calculate x which is the concentration of Chromium (VI) we substitute the value of y which is the absorbance value of sample of lake water which is 0.136. Then we get the concentration of Chromium (VI) in ppm which is 58.88 ppm. The limit of concentration of Chromium (VI) in parts per million (ppm) should contained in the water must not exceed 0.05 absorbance value. Chromium (VI) is known to be a strong oxidizing agent, which apparently has a high risk that can give an impact to humans and animals due to its carcinogenetic properties. That is why there are a lot of studies that have been done in order to determine the health- risky chromium in environmental as well as biological samples. It is naturally contained in the water but over consumed it can lead to cancer. That is why this experiment was conducted to determine whether the sample of water is either contaminated or not and whether it is safe for drinking or not.
CONCLUSIONS From the experiment of determining the Chromium (VI) concentration in water sample by using the spectrophotometer, there are few things should be highlight. That is whether the water sample is safe for drinking or not, or either at least it can be used for agricultural purpose. Based on theoretical, the concentration of Chromium (VI) that is safe for drinking is 0.05 absorbance value if the water sample has higher concentration of Chromium (VI) than 0.05, the water sample is considered contaminated. That is mean the water is not safe for drinking and maybe it can at least be used for agricultural purpose. From the experiment, it can be seen that the larger the concentration of Chromium (VI) in water sample, the larger the absorbance value which is exceed 0.05. That is mean over consuming of Chromium (VI) will lead to unhealthy body and can cause cancer.
RECOMMENDATIONS Lab coats, goggles and gloves must be wearing before do the experiment. All the process that involves the Chromium (VI) must do in the fume chamber. Besides using the spectrometer, other types of spectroscopy device could be used instead of spectrometer. When indentifying the wavelength, the reading should be taken several times instead of one only. This is to ensure the accuracy of the measurement of the absorbance that is obtained. Hence, a more accurate result regarding the concentration of chromium in the water samples can be attained. To get an ideal value, it is preferably to use a wavelength where the component absorbs substantially. To dilute the chromium (VI) solution to a 50 ml solution, it is best to use a more accurate and precise measuring apparatus instead to just add up distilled water into the 50 ml beaker. This step could cause the final volume of the solution to be more or less than the one required. As for an alternative, the needed amount of distilled water can be determined and measured first before being added to the chromium (VI). Determination of the absorbance value of each solution must be done at least 3 trials. By doing so, more accurate values can be obtained.
REFERENCES
http://eprints.utp.edu.my/1478/1/Removal_of_chromium_(VI)_from_aqueous_solution_u sing_treated_oil_palm_fibre.pdf http://www.ewg.org/chromium6-in-tap-water/executive-summary
http://www.chemguide.co.uk/analysis/uvvisible/beerlambert.html
Spectrophotometer
Das könnte Ihnen auch gefallen
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- Lab Report Guidelines and ExampleDokument6 SeitenLab Report Guidelines and ExampleAzzian AriffinNoch keine Bewertungen
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- ReferenceDokument1 SeiteReferenceAzzian AriffinNoch keine Bewertungen
- No. Pages: Table of ContentDokument18 SeitenNo. Pages: Table of ContentAzzian AriffinNoch keine Bewertungen
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- Lab ReportDokument8 SeitenLab ReportAzzian AriffinNoch keine Bewertungen
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- No. Pages: Table of ContentDokument18 SeitenNo. Pages: Table of ContentAzzian AriffinNoch keine Bewertungen
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- Test 1Dokument1 SeiteTest 1Azzian AriffinNoch keine Bewertungen
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- Membrane Test UnitDokument14 SeitenMembrane Test UnitAzzian AriffinNoch keine Bewertungen
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- Exp 2Dokument20 SeitenExp 2Azzian AriffinNoch keine Bewertungen
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- Lab Report Guidelines and ExampleDokument6 SeitenLab Report Guidelines and ExampleAzzian AriffinNoch keine Bewertungen
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- No. Pages: Table of ContentDokument18 SeitenNo. Pages: Table of ContentAzzian AriffinNoch keine Bewertungen
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- No. Pages: Table of ContentDokument18 SeitenNo. Pages: Table of ContentAzzian AriffinNoch keine Bewertungen
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Membrane SeparationDokument5 SeitenMembrane SeparationAzzian AriffinNoch keine Bewertungen
- Test 1Dokument1 SeiteTest 1Azzian AriffinNoch keine Bewertungen
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (73)
- Abstract CSTR 40lDokument13 SeitenAbstract CSTR 40lAzzian AriffinNoch keine Bewertungen
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- Tutorial 2Dokument1 SeiteTutorial 2Azzian AriffinNoch keine Bewertungen
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- Manual Calculation: °F °F T 800 °F 100lbmole/hrDokument1 SeiteManual Calculation: °F °F T 800 °F 100lbmole/hrAzzian AriffinNoch keine Bewertungen
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- Calibration CurveDokument4 SeitenCalibration CurveAzzian AriffinNoch keine Bewertungen
- Test 1Dokument1 SeiteTest 1Azzian AriffinNoch keine Bewertungen
- Test 1Dokument1 SeiteTest 1Azzian AriffinNoch keine Bewertungen
- Calibration CurveDokument4 SeitenCalibration CurveAzzian AriffinNoch keine Bewertungen
- References AaaaDokument1 SeiteReferences AaaaAzzian AriffinNoch keine Bewertungen
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- Result: Graph of Weight of Permeates Against TimeDokument1 SeiteResult: Graph of Weight of Permeates Against TimeAzzian AriffinNoch keine Bewertungen
- Test 1Dokument1 SeiteTest 1Azzian AriffinNoch keine Bewertungen
- Discussion: 2 Naoh, ODokument2 SeitenDiscussion: 2 Naoh, OAzzian AriffinNoch keine Bewertungen
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- SCO2Dokument18 SeitenSCO2Azzian AriffinNoch keine Bewertungen
- SCO2Dokument18 SeitenSCO2Azzian AriffinNoch keine Bewertungen
- Test 1Dokument1 SeiteTest 1Azzian AriffinNoch keine Bewertungen
- Lab Report Guidelines and ExampleDokument6 SeitenLab Report Guidelines and ExampleAzzian AriffinNoch keine Bewertungen
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (120)
- Lab Report Guidelines and ExampleDokument6 SeitenLab Report Guidelines and ExampleAzzian AriffinNoch keine Bewertungen
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)