Beruflich Dokumente
Kultur Dokumente
Page Rewritten Evolution
Hochgeladen von
api-894731Originalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Page Rewritten Evolution
Hochgeladen von
api-894731Copyright:
Verfügbare Formate
Notes
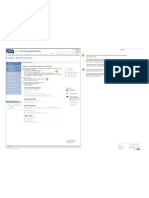
This is just a screenshot of the Manufacturers page, taken in
November, 2008.
Text Size Notes
U.S. Food & Drug Administration Search This is a rewrite of the Manufacturers page: http://www.fda.gov/cber/
manufacturer.htm.
FDA Homepage Food Drugs Medical Devices Biologics Animal & Veterinary Cosmetics Radiation-Emitting Products Combination Products
1
It uses the new page design, but the text has also been rewritten to be more web-
friendly. Specifically:
Biologics, Blood & Vaccines o The sentences are short and simple. They are in active voice. They can be skimmed
easily.
o Bullet lists create a more open page.
Biologics, Blood & Biologics, Blood & Vaccines o The most important information is presented first.
Vaccines o Hyperlinks are grouped.
Guidance, Guidance, Compliance & Regulatory Information Questions and Open Issues:
Compliance & The original page refers to both the “Manufacturers Assistance Branch” and the
The Manufacturers Assistance Branch can answer questions on many policy and Email Page “Manufacturers Assistance and Technical Training Branch”. I didn’t know which name
Regulatory procedural topics including:
Information was current, so I used the shorter of the two in this wireframe. Which is correct?
o Information on clinical investigators Print Page
o How to report an adverse event
1
Bookmark and share The page now has an announcement for a training program that closed on February 21. 2
Acts, Rules & o How to submit an application online (electronic submission)
I did not include this announcement in this wireframe.
Regulations o How to submit an Investigational New Drug Application (IND) to administer Get email updates
an investigational product to humans
Subscribe to RSS The phone numbers are the same general phone numbers shown in the Contact Us
Guidances Please contact us: module. Are there more specific numbers to use?
o Phone: 800-835-4709 or 301-827-1800 2
Establishment o E-mail: matt@cber.fda.gov Is the e-mail address an individual’s name or an acronym for the Manufacturers
Registration Assistance and Technical Training Branch? If the latter, I recommend putting it in in all
We answer questions and provide training to: CAPS, so it will look more like an acronym. This argues for using the longer branch name
o Large and small manufacturers (see paragraph 1), but which is correct?
Compliance Activities o Trade associations
Enforcement
Acts, Rules & Regulations Contact Us
Post-Market Activities Public Health Service Act 800-835-4709
301-827-1800
Rules
Imports & Exports
octma@cber.fda.gov
Comprehensive List of Laws Enforced
Code of Federal Regulations – Biologics Enforced
Center for Biologics
Evaluation and Research
1401 Rockville Pike, Suite
Guidances & SOPs 200N
Rockville, MD 20852-1448
Guidances by Topic & Year
Resources for You Manual of SOPs Media Inquiries
Consumers & Healthcare About CBER
Providers Imports & Exports
Compliance Program Guidance Manual – Imported CBER-Regulated
Industry
Products
Export Certificates
Importing Samples for Research Use Only
Other Topics
Compliance Activities
Enforcement
Post-Market Activities
Page Last Reviewed:
Page Last Updated:
Content Source:
FDA Footer
Das könnte Ihnen auch gefallen
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (121)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (400)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- Vefv1100 Vessel/S&T Heat Exchanger Standard DetailsDokument55 SeitenVefv1100 Vessel/S&T Heat Exchanger Standard Detailskmilo1978Noch keine Bewertungen
- Advanced AuditingDokument285 SeitenAdvanced AuditingMuhammad AkhtarNoch keine Bewertungen
- Enterprise Architecture StandardsDokument19 SeitenEnterprise Architecture StandardsSmitasamratNoch keine Bewertungen
- Risk Assessment Matrix ProcessDokument7 SeitenRisk Assessment Matrix ProcessEdwin CruzNoch keine Bewertungen
- Data Privacy Consent: II. Consent For Procedure(s)Dokument1 SeiteData Privacy Consent: II. Consent For Procedure(s)kNoch keine Bewertungen
- Clean Air ActDokument48 SeitenClean Air ActEphraim Gieronymus Esteban80% (10)
- School Action Plan On Gulayan Sa Paaralan ProgramDokument8 SeitenSchool Action Plan On Gulayan Sa Paaralan ProgramBea Claresse Tarape89% (9)
- To Do CurrentDokument5 SeitenTo Do Currentapi-894731Noch keine Bewertungen
- To Do CurrentDokument5 SeitenTo Do Currentapi-894731Noch keine Bewertungen
- To Do CurrentDokument5 SeitenTo Do Currentapi-894731Noch keine Bewertungen
- To Do CurrentDokument5 SeitenTo Do Currentapi-894731Noch keine Bewertungen
- To Do CurrentDokument5 SeitenTo Do Currentapi-894731Noch keine Bewertungen
- Biologics Topic Page EvolutionDokument3 SeitenBiologics Topic Page Evolutionapi-894731Noch keine Bewertungen
- To Do CurrentDokument5 SeitenTo Do Currentapi-894731Noch keine Bewertungen
- Register Blood Bank Scenario v4 Blood Topic PageDokument5 SeitenRegister Blood Bank Scenario v4 Blood Topic Pageapi-894731Noch keine Bewertungen
- Giving Blood Scenario Blood Topic PageDokument4 SeitenGiving Blood Scenario Blood Topic Pageapi-894731Noch keine Bewertungen
- To Do CurrentDokument5 SeitenTo Do Currentapi-894731Noch keine Bewertungen
- Manufacturer Page Rewritten - HTMDokument1 SeiteManufacturer Page Rewritten - HTMapi-894731Noch keine Bewertungen
- Register Blood Bank Scenario v4 Blood Topic PageDokument5 SeitenRegister Blood Bank Scenario v4 Blood Topic Pageapi-894731Noch keine Bewertungen
- To Do CurrentDokument10 SeitenTo Do Currentapi-894731Noch keine Bewertungen
- To Do CurrentDokument10 SeitenTo Do Currentapi-894731Noch keine Bewertungen
- Manufacturer Page Rewritten - HTMDokument1 SeiteManufacturer Page Rewritten - HTMapi-894731Noch keine Bewertungen
- Giving Blood Scenario Blood Topic PageDokument4 SeitenGiving Blood Scenario Blood Topic Pageapi-894731Noch keine Bewertungen
- Register Blood Bank Scenario v4 Blood Topic PageDokument5 SeitenRegister Blood Bank Scenario v4 Blood Topic Pageapi-894731Noch keine Bewertungen
- Donating Blood Page No CalloutsDokument1 SeiteDonating Blood Page No Calloutsapi-894731Noch keine Bewertungen
- Combination Topic Page OnlyDokument1 SeiteCombination Topic Page Onlyapi-894731Noch keine Bewertungen
- Giving Blood Scenario Blood Topic PageDokument4 SeitenGiving Blood Scenario Blood Topic Pageapi-894731Noch keine Bewertungen
- Giving Blood Scenario Blood Topic PageDokument4 SeitenGiving Blood Scenario Blood Topic Pageapi-894731Noch keine Bewertungen
- Combination Topic Page OnlyDokument1 SeiteCombination Topic Page Onlyapi-894731Noch keine Bewertungen
- Giving Blood Scenario Blood Topic PageDokument4 SeitenGiving Blood Scenario Blood Topic Pageapi-894731Noch keine Bewertungen
- Biologics Topic Page No Left NavDokument1 SeiteBiologics Topic Page No Left Navapi-894731Noch keine Bewertungen
- CVM WireframesDokument4 SeitenCVM Wireframesapi-894731Noch keine Bewertungen
- Giving Blood Scenario Blood Topic PageDokument4 SeitenGiving Blood Scenario Blood Topic Pageapi-894731Noch keine Bewertungen
- Giving Blood Scenario Blood Topic PageDokument4 SeitenGiving Blood Scenario Blood Topic Pageapi-894731Noch keine Bewertungen
- Giving Blood Scenario Blood Topic PageDokument4 SeitenGiving Blood Scenario Blood Topic Pageapi-894731Noch keine Bewertungen
- Biologics Topic Page No Left NavDokument2 SeitenBiologics Topic Page No Left Navapi-894731Noch keine Bewertungen
- UK - IMarEST Application Assessment ProcessDokument2 SeitenUK - IMarEST Application Assessment ProcessjeffreymacaseroNoch keine Bewertungen
- AMA Letter On Graham Cassidy Amendment FinalDokument2 SeitenAMA Letter On Graham Cassidy Amendment FinalStephen LoiaconiNoch keine Bewertungen
- Request For Irish Social Insurance Records Forms E104 and U1Dokument2 SeitenRequest For Irish Social Insurance Records Forms E104 and U1maria muela bravoNoch keine Bewertungen
- Chpts 1-6 Employee Benefits QuizDokument17 SeitenChpts 1-6 Employee Benefits QuizKevin Fernando100% (1)
- As NZS 3580.9.3-2003 Methods For Sampling and Analysis of Ambient Air Determination of Suspended ParticulateDokument3 SeitenAs NZS 3580.9.3-2003 Methods For Sampling and Analysis of Ambient Air Determination of Suspended ParticulateSAI Global - APACNoch keine Bewertungen
- Peer Dha ProjectDokument79 SeitenPeer Dha ProjectRuss LatinoNoch keine Bewertungen
- Italy Business Visa Document List PDFDokument2 SeitenItaly Business Visa Document List PDFAvinash ParavadaNoch keine Bewertungen
- The Code and Practice of Toilets in The United States of AmericaDokument6 SeitenThe Code and Practice of Toilets in The United States of AmericaFake nameNoch keine Bewertungen
- United States v. Natalya Shvets, 3rd Cir. (2015)Dokument9 SeitenUnited States v. Natalya Shvets, 3rd Cir. (2015)Scribd Government DocsNoch keine Bewertungen
- How Can We Reduce Wealth Gap Between Rich & Poor Class NoteDokument6 SeitenHow Can We Reduce Wealth Gap Between Rich & Poor Class NoteSURAJ GUPTANoch keine Bewertungen
- 2017 Form 760 InstructionsDokument56 Seiten2017 Form 760 InstructionsicanadaaNoch keine Bewertungen
- Ethics in CounselingDokument38 SeitenEthics in CounselingJobelle Cariño ResuelloNoch keine Bewertungen
- Federal BenefitsDokument180 SeitenFederal BenefitsdirtywisdomNoch keine Bewertungen
- Aurobindo Pharma Limited - 577033 - 062F202F2019 FDADokument7 SeitenAurobindo Pharma Limited - 577033 - 062F202F2019 FDAChandan ShahNoch keine Bewertungen
- Monthly Current Affairs November 2023 EnglishDokument103 SeitenMonthly Current Affairs November 2023 Englisharyankumawat40Noch keine Bewertungen
- AVERT PD AD 12 Aug 08Dokument3 SeitenAVERT PD AD 12 Aug 08scuds85Noch keine Bewertungen
- Industrial Security ManagementDokument5 SeitenIndustrial Security ManagementCarl Izan Clapero III100% (1)
- Florida's New Statutory Presumption of Undue Influence-Does It Change The Law or Merely Clarify? - The Florida Bar 2003Dokument10 SeitenFlorida's New Statutory Presumption of Undue Influence-Does It Change The Law or Merely Clarify? - The Florida Bar 2003ValSNoch keine Bewertungen
- Jakarta CourtDokument24 SeitenJakarta CourtHarish MurthiNoch keine Bewertungen
- Income ExpenditureDokument16 SeitenIncome ExpenditureSubuk T. RathodNoch keine Bewertungen
- CREW: Department of Health and Human Services: Public Affairs Firms Documents: 01/24/07: HHS/FDA FOIA ResponseDokument247 SeitenCREW: Department of Health and Human Services: Public Affairs Firms Documents: 01/24/07: HHS/FDA FOIA ResponseCREWNoch keine Bewertungen
- Code of Practice For Good PDFDokument6 SeitenCode of Practice For Good PDFbalandong4Noch keine Bewertungen
- 1ST Pta Meeting 2022-2023Dokument5 Seiten1ST Pta Meeting 2022-2023jesiebel mabliNoch keine Bewertungen