Beruflich Dokumente
Kultur Dokumente
Untitled
Hochgeladen von
Ramesh PanneerselvamOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Untitled
Hochgeladen von
Ramesh PanneerselvamCopyright:
Verfügbare Formate
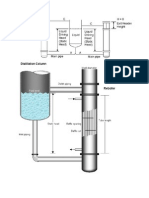
Understanding vapour pressure.(hyperphysics.phy-astr.gsu.
edu/hbase) Evaportion Ordinary evaporation is a surface phenomenon some molecules have enough kinectic energy to escape .If the container is closed ,an equilibrium is reached where an equal number of molecules return to the surface .The pressure of this equ ilibrium is called the saturation vapour pressure.In order to evaporate ,a mass of water must collect the largest heat of vaporization.so evaporation is a cool ing process.Heat of vaporization is needed to break intermolecular forces (poten tial energy associated with molecules ) and to provide the energy to expand th e gas(kinectic energy and rotational ,vibrational energy). Saturated vapour pressure The process of evaporation in a closed container will proceed until there are a s many molecules Returning to the liquid as there are escaping .at this point the vapour is Said to be saturated and the pressure of the vapour (usually expressed in mmhg) is called saturated vapour pressure. Since the molecular kinectic energy is greater at high temperature,more molecule s can escape the surface and saturated vapour pressure is correspondingly higher .if the liquid is open to the ai ,then the vapour pressure is seen as partial p ressure along which the other constituents of the air.The temperature a which va pour pressure equals atmospheric pressure.is called boiling point. Evaportion vs boiling Ordinary evaporation is a surface phenomenon- since vapor pressure is low and s ince the pressure inside the liquid is equal to atmospheric plus the liquid pr essure,bubbles of water vapor cannot form.but at the boiling point, the saturate d vapor pressure is equal To atmospheric pressure,bubbles form,and the vaporization becomes a volume pheno mena. Bolining vapour point The boiling point is defined as the temperature at which the saturted vapour pressure of aliquid is equal to Surrounding pressure.For water ,the vapor pressure reaches the standard sea leve l atm pressure of 760mmhg @ 100 deg celusius. At boiling point bubbles can form and rise since the vapor pressure overcomes atmospheric pressure. Before boili ng point atmospheric forces the liquid or exerts pressure in opposite side wher e air exerts force on liquid once in boiling point this force is counter effec ted.so molecules movement starts from inside of the vessel making it to boil.b ubbles collapse @ surface.when Vapor pressure less than atm pressure bubbles air will exert more pressure that only molecules are from surface.In boiling point vapor pressure acts as a defe nse to counter effect atmospheric pressure so no force excerted by air on liquid so bubbles form same as 10 mts of water column countereffects 760mmhg negative pressure and does t allow atm to enter the vaccum. 1)whenever vapour pressure of fluid equals surrounding pressure,it starts to b oil. 2) when vapor pressure of a substance is more @ atm conditions.It would automati cally more vapor will be in atm conditions ,when vessel is opened it will flow to atmosphere.solvents like methanol having high vapor pressure @ atm pressure i s having a great freedom to be in vaporstate as equilibrium takes place at larg e pressure of vapor or takes longer time to acheive.but for other ordinay liquid s equilibrium is fast established they don t get a chance to Be in vapor state.so li quid having high vapour pressure can considerably put large proportion of itsel f in vapor state as equilibrium is not attained quickly. 3)Boiling is a volume evaporation not a surface phenomenon. 4)when temperature increases liquids have kinectic energy so it can have lot of energy to put itself to vaporstate.at higher temperature liquids will have high
er energy to break equilibrium until next equilibrium is attained.so vapor press ure increases with temperature and decreases with temperature.so solvents can p ut large proportion of its volume in increased temperature as equilibrium vapor pressure is high.making a safety concern. 5)while boiling if we open the system to atmosphere air losses pressure or con centration, so as long as heat is given vapour pressure is high as kinetic ener gy is high now.so more liquid will be put in vapor state.but this time as syste m is opened ,system to attain equilibrium will not reverse towards low pressure liquid side .but to low pressure atmospheric side to acheieve equilibrium with liquid . 6)vapor pressure reduces with temperature.when with air mixture vapour pressure is called partial pressure.If you want to prevent vaporizing you can increase su ounding pressure so that defense system of Equilibrium vapor pressure is broken and surrounding pressure exerts force on li quid.so only equilibrium is maintained. 7) In drying beyond 100% relative humidity ,when you reduce temperature,vapour pressure suddenly reduces so water will condense from air to solid as unbound moisture as molecules has put themselves in liquid phase when vapour pressure re duces. 8)A liquid with increase pressure can boil at low boiling point. 9) A liquid at mountain will boil at low temaperature due to low surrounding prssu re but it will take longe time to cook as temperature is low. 10) Flashing can be done by introducing a liquid at boiling point or heat the su sbstance at high temperature and suddenly reducing surrounding pressure.Flashin g can be seen as bumping in reactors.
Das könnte Ihnen auch gefallen
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (400)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (345)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (74)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (121)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- Thermodynamic Properties of Water Tabulation From The lAPWS Formulation 1 995 - NistirDokument102 SeitenThermodynamic Properties of Water Tabulation From The lAPWS Formulation 1 995 - NistirViajante_santosNoch keine Bewertungen
- B.tech Chem 6-8 Aff26042010Dokument33 SeitenB.tech Chem 6-8 Aff26042010Adithya KrishnanNoch keine Bewertungen
- Refinery and Petrochemical Equipment: Distillation ColumnDokument48 SeitenRefinery and Petrochemical Equipment: Distillation ColumnFikrie MuhdNoch keine Bewertungen
- Dokumen - Tips Rate Controlled SeparationsDokument881 SeitenDokumen - Tips Rate Controlled SeparationsCátia FeijãoNoch keine Bewertungen
- 29 - Appendix 1 - Three-Phase Flash Calculation For Hydrocarbon Systems Containing WaterDokument1 Seite29 - Appendix 1 - Three-Phase Flash Calculation For Hydrocarbon Systems Containing Waterehsan_sa405Noch keine Bewertungen
- ECUST PROII Advanced Training PDFDokument118 SeitenECUST PROII Advanced Training PDFframon.chem35Noch keine Bewertungen
- Vapor Liquid Equilibria: A Review: ArticleDokument16 SeitenVapor Liquid Equilibria: A Review: ArticleLeonardo ReyesNoch keine Bewertungen
- Exp 1 Bubble Cap Distillation UnitDokument11 SeitenExp 1 Bubble Cap Distillation UnitnasuhaNoch keine Bewertungen
- Design of Cobalt Solvent Extraction Using Cyanex 272 As Extractant by Combining Mccabe Thiele Procedure and Simulation ProgramDokument46 SeitenDesign of Cobalt Solvent Extraction Using Cyanex 272 As Extractant by Combining Mccabe Thiele Procedure and Simulation Programjoseph kafumbilaNoch keine Bewertungen
- Chapter 4 - Gas AbsorptionDokument95 SeitenChapter 4 - Gas AbsorptionBoyHaha100% (7)
- Select Thermodynamic Models For Process Simulation - A Practical Guide To A Three Steps MethodologyDokument12 SeitenSelect Thermodynamic Models For Process Simulation - A Practical Guide To A Three Steps MethodologyBegenkz100% (1)
- B. Eng (Hons) Chemical Engineering: Course OutlineDokument9 SeitenB. Eng (Hons) Chemical Engineering: Course OutlinemarkNoch keine Bewertungen
- Material Balances Design Project Manufacture of Diethyl EtherDokument39 SeitenMaterial Balances Design Project Manufacture of Diethyl EthermoheedNoch keine Bewertungen
- Sulzer Fractional CrystallizationDokument16 SeitenSulzer Fractional CrystallizationHH KevinNoch keine Bewertungen
- Strategies To Maximize Ethane Recovery With High-CO FeedsDokument21 SeitenStrategies To Maximize Ethane Recovery With High-CO FeedsAditya WidiyadiNoch keine Bewertungen
- ChemEngineering - Flash EvaporationDokument3 SeitenChemEngineering - Flash EvaporationmegakiranNoch keine Bewertungen
- Spe 10127Dokument16 SeitenSpe 10127Raúl RivasNoch keine Bewertungen
- Chem 111.1 Reference Clausius Clapeyron4Dokument3 SeitenChem 111.1 Reference Clausius Clapeyron4Emrico Luiz PerezNoch keine Bewertungen
- Thermosiphon ReboilerDokument6 SeitenThermosiphon Reboilerme12m11350% (2)
- Chemical Engineering GATE 1999Dokument13 SeitenChemical Engineering GATE 1999Anonymous 8pCXXsNoch keine Bewertungen
- IV B. TECH (Chemical Engineering) I SemesterDokument14 SeitenIV B. TECH (Chemical Engineering) I SemesterPiyush AmbulgekarNoch keine Bewertungen
- 4.1 Transferencia Convectiva Entre FasesDokument4 Seiten4.1 Transferencia Convectiva Entre FasesDaniel Eduardo ValenzuelaNoch keine Bewertungen
- 3 Equilibrium PDFDokument63 Seiten3 Equilibrium PDFShuaib MohamedNoch keine Bewertungen
- CEM All Problems Till Quiz1Dokument8 SeitenCEM All Problems Till Quiz1Muhammad Irfan MalikNoch keine Bewertungen
- CH Be 3110 ProblemsDokument75 SeitenCH Be 3110 ProblemsAnkit DhalNoch keine Bewertungen
- Revista Mexicana de Ingeniería Química 1665-2738: Issn: Amidiq@xanum - Uam.mxDokument16 SeitenRevista Mexicana de Ingeniería Química 1665-2738: Issn: Amidiq@xanum - Uam.mxEllen Gracia HindrawanNoch keine Bewertungen
- Synthesis of Separation TrainsDokument22 SeitenSynthesis of Separation Trains伟铭Noch keine Bewertungen
- Si 2Dokument3 SeitenSi 2sgwala892Noch keine Bewertungen
- Amine Process Simulation Model DevelopmentDokument9 SeitenAmine Process Simulation Model DevelopmentacetilenaNoch keine Bewertungen
- Solution - Colligative Properties HLP PDFDokument24 SeitenSolution - Colligative Properties HLP PDFGOURISH AGRAWALNoch keine Bewertungen