Beruflich Dokumente
Kultur Dokumente
181 Full
Hochgeladen von
Mohammed FadhilOriginalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
181 Full
Hochgeladen von
Mohammed FadhilCopyright:
Verfügbare Formate
Paper Chromatography of Antifungal Antibiotics
Alfred Ammann and David Gottlieb Appl. Microbiol. 1955, 3(3):181.
Updated information and services can be found at: http://aem.asm.org/content/3/3/181.citation Downloaded from http://aem.asm.org/ on May 26, 2012 by guest These include:
CONTENT ALERTS
Receive: RSS Feeds, eTOCs, free email alerts (when new articles cite this article), more
Information about commercial reprint orders: http://journals.asm.org/site/misc/reprints.xhtml To subscribe to to another ASM Journal go to: http://journals.asm.org/site/subscriptions/
Paper Chromatography of Antifungal Antibiotics
ALFRED AMMANN
AND
DAVID GOTTLIEB
Department of Horticulture, University of Illinois, Urbana, Illinois
Received for publication January 3, 1955
Paper chromatography is very helpful in the identification and comparison of antibiotics, but very few results from the application of this technique have been published so far. The awakening of interest in the search for antibiotics which are active against fungi pathogenic to plants and animals makes the investigator aware of the scant information that is available to help characterize such agents. A knowledge of the Rf values of the known antifungal agents would help advance the search for such therapeutic materials. Often these values, in conjunction with the biological inhibitory activities, are the only criteria by which one can determine whether the unknown agent appears to be a new material. When the Rf data are obtained for shake cultures, one can eliminate many previously described antibiotic-producing organisms from further study, thus
allowing more research to be applied to antibiotics which are new and different. The studies reported in this paper should serve as a guide to the identification of some of the known antifungal antibiotics.
MATERIALS AND METHODS The one-dimensional, ascending paper chromatogram technique was used. The paper strips (18 x 35 cm) were cut from large sheets of Whatman filter paper No. 1 (47 x 57 cm) by dividing the long dimension into widths of 18 cm and then cutting these sections to a length of 35 cm. Solutions of the antibiotic were placed 3 cm from the lower edge of the strip with a micropipette in 5-lambda (0.005-ml) portions until the desired quantity of substance had been added. The amounts placed on the paper varied with the sensitivity
Downloaded from http://aem.asm.org/ on May 26, 2012 by guest
TABLE 1. Details of Pyrex dish technique
Test Organism
Antibiotics
Agar
Incubation Incubation Time Temp
hr C
Spore Suspension
Penicillium oxalicum 99*
Filipin Nystatin Fradicin Ascosin Thiolutin Rimocidin sulfate Trichomycin
Trypticase soy agart
18-24
26
5 ml with a light transmission of 50% in 120 ml agar
Glomerella cingulata 100t
Bacillus subtilis Cohn 23
Antimycin A
Actinomycin Gliotoxin Nigericin Streptothricin
sulfate
Trypticase soy agart
Emerson's agar
40 18
26 30
5 ml with a light transmission of 50% in 120 ml agar
400,000 spores per ml agar
Candida albicans 40
Candicidin A
Emerson's agar
18
30
Endomycin
1 ml 1-day-old Emerson's broth culture in 120 ml agar
Saccharomyces sp. 45
Cycloheximide
Emerson's agar
18-24
* Numbers for test organisms correspond to numbers used in the collection of D. Gottlieb, versity of Illinois, Urbana, Illinois. t Baltimore Biological Laboratories, Baltimore, Md. t To prevent bacterial contamination 100 jug/ml Chloramphenicol were included in the agar. Yeast extract, 1 g; bactopeptone, 4 g; beef extract, 4 g; NaCl, 2.5 g; cerelose, 10 g; agar, 15 to 20 g; distilled water, 1000 ml Probably S. pastorianus. 181
1 ml 1-day-old Emerson's broth culture in 120 ml agar Horticulture Field Laboratory, Uni30
182
ALFRED AMMANN AND DAVID GOTTLIEB
tom of cylindrical glass jars (25 x 45 cm). After the spots of solution on the paper strip had dried, the sheet was suspended in the jar so that approximately 1 cm dipped into the solvent system. The papers were then developed at 26 C until the solvent front had moved up about 27 cm. The strips were then air-dried and placed on seeded assay plates for 10 to 15 minutes.
of the test organism, the solvent for the antibiotic, and the solvent system used for developing the chromatogram. Four materials were tested on each strip. Eight hundred ml of solvent mixture were placed in the botTABLE 2. Solvent systems and their development time
Number
Solvent Systems
Development Time
hr
I II III IV V VI
VII
Water saturated n-Butanol n-Butanol:acetic acid:H20 (2:1:1) n-Butanol: pyridine:H20 (1:0.6:1) 3% Ammonium chloride 50% hydrous acetone Benzene: acetic acid: H20 (2:2: 1) (organic layer) n-Butanol saturated water
15-16 15-16 15-16 2-3 6-7 7-10
8-10
The position of the antibiotic on the paper was determined in the following manner: The Pyrex dish technique (Peterson and Reineke, 1950) was used and 120 ml of a seeded agar particularly suited to each organism were placed in the sterile dish. All plates were prepared immediately before putting the paper strip on the seeded agar. After incubation, clear zones occurred wherever the antibiotic had diffused from the paper into the agar. The distance that the solvent system had moved in the same time was measured on the original
on
Downloaded from http://aem.asm.org/ on May 26, 2012 by guest
TABLE 3. Amount of antibiotics used
Antibiotic
paper
strips
Purity
ConcentrationSolution and Solvent of Stock
Minimum and Maximum Amounts in pAg Brought on Paper Strips for Solvent System No. :* I 1 II III IV VII v
II
IVI
Actinomycin............. Crystals
Antimycin A Crystals
1 mg/ml in acetone
2-3
2-3
2-3
2-50
2-3
2-50
2-10
100,ug/ml in
tone
1
ace-
0.2-0.5
mg/ml in meth5-100 anol 100 Jg/ml in meth- 0.2-0.25 0.3 0.3 0.3 anol 10 lg/ml in meth0.05 0.1 anol Ascosin ...I Crude 1 mg/ml in meth75 25 25-75 75 25-75 75 anol Candicidin A.............. Crude, 6000 u/ 1 mg/ml in 50% 100-150 100 300 400 75-100 150 mg ethanol Cycloheximide ............ Crystals 100 jug/ml in dis0.2-1 0.2-1 0.2-1 0.2-1 0.2-0.5 0.2-1 tilled water Endomycin ............... Crude 1000 u/mg 1 mg/ml in meth200 200 200 200 200 200 anol ....Crude 500 u/mg Filipin 1 mg/ml in meth- 75-100 75-100 75 75 50-75 50-75 50-100 anol Fradicin .................. Crystals 1 mg/ml in ethyl- 40-75 40-75 40-75 50-75 25-100 40-75 75-100 ene dichloride Gliotoxin................ Crystals 1 mg/ml in acetone 15-50 15-50 15-50 10-15 15-50 15-50 Nigericin ................. Crystals 1 mg/ml in meth- 25-50 25-50 25-50 25-50 5-25 50 anol 100 ug/ml in meth1 anol Nystatin .................. Crude 400,ug/ml in meth- 30-40 30-40 40 20-40 20-40 40 anol Rimocidin sulfate. Crystals 1 mg/ml in meth25 25-100 25-100 75-100 25-75 50-75 anol Streptothricin sulfate. Crystals 1 mg/ml in 50% 50 50 20-50 50 20 50 methanol Thiolutin Crude, 660 'Ag/ 1 mg/ml in meth- 50-75 50-75 50-75 25-75 75 75 25-35 mg anol Trichomycin 3200 u/mg 1 mg/ml in ethyl5-50 5 2-5 5-25 10
ene
100
lAg/ml in ethylene
1-2
_I
Composition of these systems is given in table 2.
PAPER CHROMATOGRAPHY OF ANTIFUNGAL ANTIBIOTICS
TABLE 4. Rf values* of 16 antibiotics
Antibiotic
183
Rf Value in Solvent System No. :t
I
II III IV
VI
VII
With antifungal and antibacterial activity Actinomycin ................................
0.96
0.69 0.90 0.95
0.94
0.93 0.95 0.98
0.96
0.86 0.96 0.98
0.00-0.21
0.00 0.54 0.00
0.96
0.88 0.88 0.86
0.99
0.00 0.98 0.00 & 1.00
0.53t or
0.70
Endomycin ................................. Gliotoxin ................................... Nigericin ................................... Streptothricin sulfate .. ..................... Thiolutin ...................................
With antifungal activity only Antimycin A ................................ Cycloheximide ...................0...........
0.28 0.48
0.00
0.83
0.13
0.90
0.09
0.93
0.94
0.28
0.18
0.77 0.86
0.00
1.00 1.00
0.93 .90
0.91 0.84
0.98 0.96
0.95 0.85
0.97 0.96
0.96
0.00
0.87 0.00
0.20
0.00
0.91
0.72
0.96
0.18
Filipin ....................................
Fradicin ...................................
Ascosin .................................... Candicidin A .................................. Trichomycin ............
0.94
0.00 0.00
0.00
0.36-0.67
0.43-0.73 0.38-0.69 0.68
0.91
0.89
0.00
0.00
0.07
Downloaded from http://aem.asm.org/ on May 26, 2012 by guest
0.33 0.44 0.35
0.88 0.86
0.79
0.80
0.85
0.76
0.73 0.77
0.00
?
0.00
Nystatin ............ Rimocidin sulfate ............
0.25
0.39
0.76
0.84
0? .82
0.00
0.00
* Average of at least two determinations. t Composition of these systems is given in table 2. t With amounts of 2 to 10 ,ug. With amounts of 0.2 to 0.5 ,ug.
paper strip. An Rf value was calculated as the ratio of movement of the antibiotic to that of the solvent front. Seven solvent systems. were used for 15 different antifungal agents, 6 of which also show antibacterial activity. These antibiotics were. actinomycin (Waksman and Tishler, 1942; obtained from Hoffmann-La Roche Inc., Nutley, New Jersey); antimycin A (Dunshee et al., 1949; obtained from University of Wisconsin, Madison); ascosin (Hickey et at., 1952; obtained from Commercial Solvents Corp., Terre Haute, Indiana); candicidin A (Lechevalier et at., 1953; obtained from Rutgers University, New Brunswick, New Jersey); cycloheximide (Whiffen, 1948; obtained from Upjohn Co., Kalamazoo, Michigan); endomycin (Gottlieb et al., 1951; obtained from Upjohn Co., Kalamazoo, Michigan); filipin (Gottlieb et al.; produced at the University of Illinois, Urbana, Illinois); fradicin (Swart et al., 1950; obtained from Commerical Solvents Corp., Terre Haute, Indiana); gliotoxin (Brian and Hemming, 1945; obtained from the University of Illinois, Urbana, Illinois); nigericin (Harned et al., 1951; obtained from Commercial Solvents Corp., Terre Haute, Indiana); nystatin (Hazen and Brown, 1951; obtained from E. R. Squibb & Son, New Brunswick, New Jersey); rimocidin sulfate (Davisson et al., 1951; obtained from Chas. Pfizer & Co., Inc., Brooklyn, New York); streptothricin sulfate (Waksman and Woodruff, 1942; obtained from Upjohn Co., Kalamazoo, Michigan); thiolutin (Seneca et al., 1952; obtained from Chas. Pfizer & Co., Inc., Brooklyn, New York); trichomycin (Hosoya et al.,
1952; obtained from National Institute of Health, Tokyo, Japan). All further information concerning the test organisms, solvent systems, concentrations of different antibiotics, and so forth, is given in tables 1, 2, and 3.
RESULTS AND DISCUSSION The Rf values of the 15 antibiotics are given in table 4. In a few cases certain solvent systems were unsatisfactory for specific antibiotics. For example, ascosin, candicidin A, and fradicin in 50 per cent acetone gave Rf values which varied over a rather wide range. Nystatin and rimocidin sulfate were inactive when placed in 3 per cent ammonium chloride. Nigericin, even though crystalline, always gave two spots in the
benzene-acetic acid-water system. The results in table 4 provided the information for preparing two flow sheets (figures 1 and 2), on which most of the 15 antibiotics can be separated on the basis of their Rf values. In some cases the differences are not very pronounced and other properties of the material must also be considered in order to determine the identity of the antibiotic. Separation of the ascosin-candicidin-trichomycin and nystatin-rimocidin groups (Okami et al., 1954; Vining et al., 1954) was very difficult with the solvent systems used in this study and ascosin could not be separated from trichomycin. Further research with other solvent systems will certainly lead to a better separation of these two groups and their members.
184
ALFRED AMMANN AND DAVID GOTTLIEB
3% NH4Cl
Rf values 0.00-0.40
Rf values 0.41-1.00 Gliotoxin
Actinomycin Endomycin Nigericin
Streptothricin
sulfate
Downloaded from http://aem.asm.org/ on May 26, 2012 by guest
Rf vf 0.00
Rf values
0.95-1.00
Rf values
0.00
Rf value
0.98
Endomycin Nigericin
Actinomycin Nigericin Thiolutin
Streptothricin
Gliotoxin
sulfate
n-Butanol saturated water
Rf VE 0.69
Rf value 0.95
Rf values 0.00-0.35
Nigericin*
Rf values 0.40-0.80
Actinomycin Thiolutin
Endomycin
Nigerici,n
50% hydrous
acetone
Rt value
0.77 Thiolutin
Rf value 0.96 c
Actinomycin
on
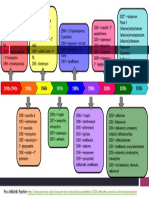
* In 50% hydrous acetone Rf value is 0.86. FIG. 1. Flow sheet for the separation of six antibiotics with antifungal and antibacterial activity in five solvent systems.
the basis of their RE values
In general, the paper chromatography technique has been helpful in establishing the identity of these antibiotics. But it is even more important that the information can be used to establish differences. This is especially necessary in the search for new antibiotics, for all other known materials must be "ruled out." Consistent differences among known materials and the
unknown, when run under the same conditions, would indicate the presence of a new material. While paper chromatography is a useful technique, it is not a specific criterion, for it sometimes fails to differentiate some closely related antibiotics. It can best be used in combination with the other methods of differentiation available to the microbiologist and chemist.
PAPER CHROMATOGRAPHY OF ANTIFUNGAL ANTIBIOTICS
Benzene:acetic acid:H20 (2:2:1) (organic layer)
,~~~~~~~~~~~~~~~~~~
185
Rf values 0.00-0.30
Ascosin Candicidin A Trichomycin Nystatin Rimocidin sulfate Filipin Water saturated n-Butanol
Rf values 0.70-1 .00
Cycloheximide Antimycin A Fradicin
Downloaded from http://aem.asm.org/ on May 26, 2012 by guest
Rf value 0.91
Rf values 0.00-0.60
Rf values 0.00
Antimycin A Fradicin
Filipin
Ascosin
Candicidin A Trichomycin Nystatin Rimocidin sulfate 50% acetone Rf values 0.38-0.73
Rf value 0.87
Cycloheximide
n-Butanol:acetic acid:H20 (2:1:1)
Rf values 0.82-1.00
Nystatin Rimocidin sulfate
/
Rf value 0.85
Fradicin
Rf value 0.98
Ascosin Candicidin A
Trichomycin
Antimycin A
Rf values
0.33-0.35
Ascosin Trichomycin
Rf value 0.44 Candicidin A
Rf value 0.25
Rf value 0.39
Rimocidin sulfate
on the basis of their
Nystatin
FIG. 2. Flow sheet for the separation of nine antibiotics with antifungal activity systems.
Rf values in five solvent
SUMMARY The Rf values of 15 antifungal antibiotics (6 of which show also antibacterial activity) in various solvent systems have been described. Flow sheets have been constructed for the separation of most of these agents on the basis of these data.
REFERENCES
BRIAN, P. W., AND HEMMING, H. G. 1945 Gliotoxin, a fungistatic metabolic product of Trichoderma viride. Ann. Appl. Biol., 32, 214-220.
DAVISSON, J. W., TANNER, F. W., JR., FINLAY, C. A., AND SOLOMONS, I. A. 1951 Rimocidin, a new antibiotic. Antibiotics & Chemotherapy, 1, 289-290. DUNSHEE, B. R., LEBEN, C. KEITT, G. W., AND STRONG, F. M. 1949 The isolation and properties of antimycin A. J. Am. Chem. Soc., 71, 2436-2437. GOTTLIEB, D., AMMANN, A., AND CARTER, H. E. 1955 A new antifungal agent, filipin. Plant Dis. Rep., 39, 219. GOTTLIEB, D., BHATTACHARYYA, P. K., CARTER, H. E., AND ANDERSON, H. W. 1951 Endomycin, a new antibiotic. Phytopathology, 41, 393-400. HARNED, R. L., HIDY, P. H., CORUM, C. J., AND JONES, K. L. 1951 Nigericin, a new crystalline antibiotic from an un-
186
J. M. SHENEMAN AND R. N. COSTILOW
identified Streptomyces. Antibiotics & Chemotherapy, 1, 594-596. HAZEN, E. L., AND BROWN, R. 1951 Fungicidin, an antibiotic produced by a soil actinomycete. Proc. Soc. Exptl. Biol. Med., 76, 93-97. HICKEY, R. J., CORUM, C. J., HIDY, P. H., COHEN, I. R., NAGER, U. F. B., AND KROPP, E. 1952 Ascosin, an antifungal antibiotic produced by a streptomycete. Antibiotics & Chemotherapy, 2, 472-483. HOSOYA, S., KOMATSU, N., SOEDA, M., YUWAGUCHI, T., AND SONODA, Y. 1952 Trichomycin, a new antibiotic with trichomonadicidal and antifungal activities. J. Antibiotics (Japan), 5, 564-566. LECHEVALIER, H., ACKER, R. F., CORKE, C. T., HAENSELER, C. M., AND WAKSMAN, S. A. 1953 Candicidin, a new antifungal antibiotic. Mycologia, 45, 155-171. OKAMI, Y., UTAHARA, R., NAKAMURA, S., AND UMEZAWA, H. 1954 Studies on antibiotic actinomycetes, IX. On Streptomyces producing a new antifungal substance mediocidin and antifungal substances of fungicidin-rimocidin-chromin group, eurocidin group and trichomycin-ascosin-candicidin group. J. Antibiotics, Ser. A (Japan), 7, 98-103. PETERSON, D. H., AND REINEKE, L. M. 1950 A paper chro-
matographic technique and its application to the study of
new
SENECA, H., KANE, J. H., AND ROCKENBACH, J. 1952 Bactericidal, protozoidal and fungicidal properties of thiolutin. Antibiotics & Chemotherapy, 2, 357-360. SWART, E. A., ROMANO, A. H., AND WAKSMAN, S. A. 1950 Fradicin, an antifungal agent produced by Streptomyces fradiae. Proc. Soc. Exptl. Biol. Med., 73, 376-378. VINING, L. C., TABER, W. A., AND LECHEVALIER, H. A. 1954 The antifungal antibiotics of the candicidin type. Huitieme
et communications parvenus avant le congres, aux sections 21 A 27, 106-110.
antibiotics. J. Am. Chem. Soc., 72, 3598-3603.
congres international de botanique, Paris. 1954 Rapports
WAKSMAN, S. A., AND TISHLER, M. 1942 The chemical nature of actinomycin, an antimicrobial substance produced by Actinomyces antibioticus. J. Biol. Chem., 142, 519-528. WAKSMAN, S. A., AND WOODRUFF, H. B. 1942 Streptothricin, a new selective bacteriostatic and bactericidal agent, particularly active against gram-negative bacteria. Proc. Soc. Exptl. Biol. and Med., 49, 207-210. WHIFFEN, A. J. 1948 The production, assay, and antibiotic activity of actidione, an antibiotic from Streptomyces griseus. J. Bacteriol, 56, 283-291.
Downloaded from http://aem.asm.org/ on May 26, 2012 by guest
Sorbic Acid
as a
Preservative for Sweet Cucumber Pickles'
J. M. SHENEMAN AND R. N. COSTILOW
Department of Microbiology and Public Health, Michigan State College, East Lansing, Michigan Received for publication January 10, 1955
The preservation of sweet cucumber pickles may be accomplished by maintaining sugar and acetic acid concentrations at sufficient levels to inhibit all organisms. However, this is not possible in the manufacture of low Baum6, low acid sweet pickles, and therefore it is necessary either to pasteurize such products or to add sodium benzoate. Recent work by Gooding (1945), Smith and Rollin (1954) and Deuel et al. (1954a, b) indicates that sorbic acid (2,4-hexadienoic acid) may be more efficient and less toxic as a food preservative than sodium benzoate. Melnick et al. (1954) found that sorbic acid would not prevent the growth of molds in cheese when the initial contamination was great. Therefore, it could not be used to preserve cheese prepared under unsanitary conditions. These studies have resulted in the tentative acceptance of this agent as a harmless food preservative by the U. S. Food and Drug Administration. Phillips and Mundt (1950) and Jones and Harper (1952) reported that 0.1 per cent sorbic acid would prevent the growth of surface yeasts in cucumber fermentations. Emard and Vaughn (1952) found sorbic acid to inhibit many species of microorganisms, including yeasts, molds and bacteria. A concentration of 0.07
l Journal Article No. 1706.
per cent at a pH of 5.0 to 5.5 was sufficient to inhibit the catalase positive organisms but allowed the catalase negative group to grow rapidly. The inhibitory action was related to the pH of the medium; as the pH decreased, the efficiency of inhibition increased. Phillips and Mundt (1950) reported that the use of 0.1 per cent sorbic acid in cucumber fermentations had no adverse effect on the flavor of the finished pickles. Smith and Rollin (1954) noted that concentrations much larger than 0.1 per cent were necessary to be detected in the flavor of cheese. Due to the apparent desirability of sorbic acid over sodium benzoate as a food preservative, a study was instigated to determine the efficiency of this compound in the preservation of sweet cucumber pickles. This paper presents the results of studies of the inhibitory effect of sorbic acid in combination with sucrose and acetic acid on yeasts from spoiled pickles and on lactobacilli. Experiments were conducted both in laboratory media and in sweet pickles.
EXPERIMENTAL METHODS
The yeast cultures used in this study were three strains of "spoiled pickle yeasts": SPY-15, SPY-21 and SPY-29. They were isolated and described by Bell anid
Das könnte Ihnen auch gefallen
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- Senior Research Scientist Biochemist in NYC NJ Resume Renee ChabinDokument3 SeitenSenior Research Scientist Biochemist in NYC NJ Resume Renee ChabinReneeChabinNoch keine Bewertungen
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- Salivary MucocelesDokument3 SeitenSalivary MucocelesnsatriotomoNoch keine Bewertungen
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Etanol BacteriaDokument38 SeitenEtanol BacteriaWida YantiNoch keine Bewertungen
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- Conquering Methicillin Resistant Staphylococcus Aureus: Perspectives and Treatment OptionsDokument7 SeitenConquering Methicillin Resistant Staphylococcus Aureus: Perspectives and Treatment OptionsInternational Medical PublisherNoch keine Bewertungen
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- Antibiotic Guidelines C Diff & PID Amended 05.08.11Dokument26 SeitenAntibiotic Guidelines C Diff & PID Amended 05.08.11mikike13Noch keine Bewertungen
- Texila American University: Article Review GuidelineDokument10 SeitenTexila American University: Article Review GuidelinecindyNoch keine Bewertungen
- Industrial Production of Beta-Lactam AntibioticsDokument8 SeitenIndustrial Production of Beta-Lactam AntibioticsSyarif AlmubarakNoch keine Bewertungen
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (400)
- Pharmacodynamics of AntibioticsDokument28 SeitenPharmacodynamics of AntibioticsHazimMahmoudDarwishNoch keine Bewertungen
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Interpretative Reading of The AntibiogramDokument40 SeitenInterpretative Reading of The AntibiogramJonathan PimientoNoch keine Bewertungen
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- Antibiotic Cross-Sensitivity ChartDokument1 SeiteAntibiotic Cross-Sensitivity Chartsuper0113Noch keine Bewertungen
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Solanum Nigrum With Dynamic Therapeutic RoleDokument7 SeitenSolanum Nigrum With Dynamic Therapeutic RoleFadli SukandiarsyahNoch keine Bewertungen
- Rapid Detection ESBLDokument7 SeitenRapid Detection ESBLVenny PatriciaNoch keine Bewertungen
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- Thesis Proposal Saccharum SpontaneumDokument6 SeitenThesis Proposal Saccharum SpontaneumApril Mergelle LapuzNoch keine Bewertungen
- Daftar Harga Apotek MEGAHDokument96 SeitenDaftar Harga Apotek MEGAHWidya Gesfa SariNoch keine Bewertungen
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- OmfalitisDokument11 SeitenOmfalitisRustinaNoch keine Bewertungen
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (345)
- Chap 1 2.0Dokument6 SeitenChap 1 2.0Landhel Galinato LoboNoch keine Bewertungen
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (74)
- Factors Contributing To Irrational Use of AntibioticsDokument2 SeitenFactors Contributing To Irrational Use of AntibioticsgoyeniranNoch keine Bewertungen
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- Pharmacology in RehabilitationDokument682 SeitenPharmacology in RehabilitationDiana Mesquita100% (1)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- 1) Each Question Carries 2 Marks. 2) Every Correct Answer Has Weightage of 2 MarksDokument482 Seiten1) Each Question Carries 2 Marks. 2) Every Correct Answer Has Weightage of 2 MarksAyushmanNoch keine Bewertungen
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- Antimicrobial Susceptibility Testing (AST)Dokument41 SeitenAntimicrobial Susceptibility Testing (AST)summiya100% (1)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- 21 Cephalosp 2015 Mandell Douglas and Bennett S Principles and PracticeDokument20 Seiten21 Cephalosp 2015 Mandell Douglas and Bennett S Principles and PracticeHelen DyNoch keine Bewertungen
- Antibiotic Approval TimelineDokument1 SeiteAntibiotic Approval TimelineMartin CuellarNoch keine Bewertungen
- Case Study Worksheet EndocarditisDokument6 SeitenCase Study Worksheet EndocarditisSharlee StoneNoch keine Bewertungen
- AQA GCSE Science Biology Keeping HealthyDokument30 SeitenAQA GCSE Science Biology Keeping HealthySteve BishopNoch keine Bewertungen
- Malhotra 2012Dokument8 SeitenMalhotra 2012Noor FatimahNoch keine Bewertungen
- 147 CinnamomunDokument460 Seiten147 CinnamomunHijriyAhHanbinNoch keine Bewertungen
- Antibiotic Therapy: The Pneumonia PanaceaDokument3 SeitenAntibiotic Therapy: The Pneumonia PanaceabobbyramakantNoch keine Bewertungen
- Botany ProDokument44 SeitenBotany ProJishnu ANoch keine Bewertungen
- Hospital Acquired PneumoniaDokument23 SeitenHospital Acquired Pneumoniadarmarianto100% (1)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (121)
- მუცლის-ტიფი სალმონელოზიDokument50 Seitenმუცლის-ტიფი სალმონელოზიგიორგი კოჩაძეNoch keine Bewertungen
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)