Beruflich Dokumente
Kultur Dokumente
Lattice
Hochgeladen von
Reejo PaulOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Lattice
Hochgeladen von
Reejo PaulCopyright:
Verfügbare Formate
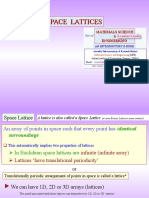
SPACE LATTICES
An array of points such that every point has identical
surroundings
In Euclidean space infinite array
We can have 1D, 2D or 3D arrays (lattices)
Space Lattice
Translationally periodic arrangement of points in space is called a lattice
or
A lattice is also called a Space Lattice
Note: points are drawn with finite size for clarity in reality they are 0D (zero dimensional)
1D Lattices
Construction of a 1D lattice 1D Lattices
Let us construct a 1D lattice starting with two points
The point on the right has one to the left and hence by the requirement of identical surrounding
the one of the left should have one more to the left
By a similar argument there should be one more to the left and one to the right
This would lead to an infinite number of points
In 1D spherical space a lattice can be finite!
The infinity on the sides would often be left out from schematics
1D Lattices
In 1D there is only one kind of lattice
This lattice can be described by a single lattice parameter (a)
To obtain a 1D crystal this lattice has to be decorated with a motif
The unit cell for this lattice is a line segment of length a
a
Starting with a point the lattice translation vector (basis vector) can generate the lattice
Note: Basis vector should not be confused with the basis ( the motif)
Click here to see how symmetry operators generate the 1D lattice
How can make some 1-D crystals out
of the lattice we have constructed
Click here
2D Lattices
2D Lattices
2D lattices can be generated with two basis vectors
They are infinite in two dimensions
There are five distinct 2D lattices:
1 Square
2 Rectangle
3 Centered Rectangle
4 120 Rhombus
5 Parallelogram (general)
To simplify matters:
In this set of slides we will NOT consider symmetries with translation built into them (e.g. glide reflection)
- - - - - - - - - - - - - - - - -
- - - - - - - - - - - - - - - - -
- - - - - - - - - - - - - - - - -
- - - - - - - - - - - - - - - - -
- - - - - - - - - - - - - - - - -
- - - - - - - - - - - - - - - - -
- - - - - - - - - - - - - - - - -
- - - - - - - - - - - - - - - - -
- - - - - - - - - - - - - - - - -
- - - - - - - - - - - - - - - - -
a
2D Lattices
Two basis vectors generate the lattice
There are three lattice parameters which describe this lattice
One angle: o
Two distances: a, b
= 90 in the current example
o
b
a
Four (4) Unit Cell shapes in 2D can be used for 5 lattices as follows:
Square (a = b, o = 90)
Rectangle (a, b, o = 90)
120 Rhombus (a = b, o = 120)
Parallelogram (general) (a, b, o)
It is clear some of them require more parameters to describe than others
Some of them have special constraints on the angle
Can we put them in some order?
The next slide defines a parameter called terseness to order them.
Square (p = 2, c = 2, t = 1)
a = b
o = 90
Rectangle (p = 3, c = 1 , t = 2)
a = b
o = 90
Parallelogram (p = 3, c = 0 , t = 3)
a = b
o
Progressive relaxation of the constraints on the lattice parameters amongst the FIVE 2D lattice shapes
Rhombus (p = 2, c = 2, t = 1)
a = b
o = 120
I
n
c
r
e
a
s
i
n
g
n
u
m
b
e
r
t
Note how the Square and the Rhombus are in the same level
p = number of independent parameters = (p e)
(discounting the number of =)
c = number of constraints (positive = some number)
t = terseness = (p c)
(is a measure of the expenditure on the parameters
E.g.
for Square: there are 3 parameters (p)
and
1 = amongst them (e)
p = (p e) = (3 1) = 2
Now let us consider the 5 lattices one by one
Square Lattice
Why put rotational symmetry elements onto a lattice?
(arent lattices built just out of translation?)
b a =
Lattice parameters: a = b, o = 90
Unit Cell with
Symmetry elements
(rotational) overlaid
Rotational + Mirrors
1
4mm
Symmetry
Rotational + Mirrors
4mm
Symmetry
A note on the symmetry
This (4mm) is the symmetry of the square lattice
Crystals based on the square lattice can have lower symmetry than the lattice itself
If the crystal based on the square lattice has 4mm or 4 symmetry then the crystal will be
called a Square Crystal (else not)
Rectangle Lattice
The shortest lattice translation vector (a < b)
Lattice parameters: a, b, o = 90
Unit Cell with
Symmetry elements
(rotational) overlaid
Rotational + Mirrors
2
2mm
Centred Rectangle Lattice
Lattice parameters: a, b, o = 90
Continued
Unit Cell with
Symmetry elements
(rotational) overlaid
3
Rotational + Mirrors
We will see the utility of the shortest
lattice translation vector in the topic on
dislocations
( )
2
a b +
Centred Rectangular Lattice
We have chosen a different unit cell but this does not
change the structure!
It still remains a centred rectangular lattice
Shape of Unit Cell does not
determine the lattice or the
crystal!!
2mm
120 Rhombus Lattice
Lattice parameters: a = b, o = 120
Unit Cell with
Symmetry elements
(rotational) overlaid
Q: I have seen a different representation of the
same unit cell WITHOUT the 6-folds. How come?
Continued
4
a b +
Rotational + Mirrors
120 Rhombus Lattice
6mm
The Hexagon shaped cell
Often one might see a cell in the form of a hexagon:
This is not a conventional cell (as it is not in the shape of a parallelogram)
This is actually a combination of 3 cells
This cell brings out the hexagonal symmetry of the lattice
It is triply non-primitive (3 lattice points per cell)
1/3 contribution to cell
1/3 6 = 2
1 (full) contribution to cell
Parallelogram Lattice
Lattice parameters: a, b, o = 90 Lattice parameters: a, b, o = 90
Unit Cell with
Symmetry elements
overlaid
There are no mirrors in parallelogram lattice
5
2
Lattice Symmetry Shape of UC Lattice Parameters
1. Square 4mm 1. Square
(a = b , o = 90)
2. Rectangle 2mm 2. Rectangle
(a = b, o = 90)
3. Centred Rectangle 2mm "
(a = b, o = 90)
4. 120 Rhombus 6mm 3. 120 Rhombus (a = b, o = 120)
5. Parallelogram 2 4. Parallelogram (a = b, o general value)
Summary of 2D lattices
Lattice Simple Centred
Square
Rectangle
120 Rhombus
Parallelogram
Every lattice that you can construct is
present somewhere in the list
the issue is where to put them!
Shows the equivalence
Why are some of the possible 2D lattices missing?
We had seen that there is a rectangle lattice and a centred rectangle lattice.
The natural question which comes to mind is that why are there no centred
square, centred rhombus and centred parallelogram lattices?
We have already answered the question regarding the centred square lattice.
(However, we will repeat the answer here again).
We will also answer the question for the other cases now.
This is nothing but a square lattice viewed at 45!
Centred square lattice = Simple square lattice
The case of the centred square lattice
Hence this is not a separate case
Based on size the smaller blue cell (with half the area) is preferred
Note that the symmetries
of are that of the square
lattice
4mm
The case of the centred rhombus lattice
Centred rhombus lattice = Simple rectangle lattice
Based on size the smaller green cell (with half the area) is preferred
Hence this is not a separate case
Note that the symmetries
of the centred rhombus
lattice are identical to the
rectangle lattice (and not
to the rhombus lattice)
The case of the centred parallelogram lattice
Centred parallelogram lattice = Simple parallelogram lattice
Based on size the smaller green cell (with half the area) is preferred
Hence this is not a separate case
Note that the symmetries
are that of the
parallelogram lattice
2
How can make some 2-D crystals out
of the lattices we have constructed
Click here
3D Lattices
3D Lattices
3D lattices can be generated with three basis vectors
They are infinite in three dimensions
3 basis vectors generate a 3D lattice
The unit cell of a general 3D lattice is described by 6 numbers (in special cases all these
numbers need not be independent) 6 lattice parameters
3 distances (a, b, c)
3 angles (o, |, )
A derivation of the 14 Bravais lattices or the existence of 7 crystal systems will not be shown in this introductory course
There are 14 distinct 3D lattices which come under 7 Crystal Systems
The BRAVAIS LATTICES (with shapes of unit cells as) :
Cube (a = b = c, o = | = = 90)
Square Prism (Tetragonal) (a = b = c, o = | = = 90)
Rectangular Prism (Orthorhombic) (a = b = c, o = | = = 90)
120 Rhombic Prism (Hexagonal) (a = b = c, o = | = 90, = 120)
Parallelepiped (Equilateral, Equiangular)
(Trigonal) (a = b = c, o = | = = 90)
Parallelogram Prism (Monoclinic) (a = b = c, o = = 90 = |)
Parallelepiped (general) (Triclinic) (a = b = c, o = | = )
To restate:
the 14 Bravais lattices have 7 different Symmetries
(which correspond to the 7 Crystal Systems)
Shape of UC Used as UC for crystal: Lattice Parameters
Cube Cubic (a = b = c, o = | = = 90)
Square Prism Tetragonal (a = b = c, o = | = = 90)
Rectangular Prism Orthorhombic (a = b = c, o = | = = 90)
120 Rhombic Prism Hexagonal (a = b = c, o = | = 90, = 120)
Parallelepiped
(Equilateral,
Equiangular)
Trigonal (a = b = c, o = | = = 90)
Parallelogram Prism Monoclinic (a = b = c, o = = 90 = |)
Parallelepiped (general) Triclinic (a = b = c, o = | = )
Important Note:
do NOT confuse the shape of the unit cell with the crystal systems
(as we have already seen we can always choose a different unit cell for a given
crystal)
Building a 3D cubic lattice
Click here to visualize a step by step construction
Each vertex of the cube is a lattice point
(no points are shown for clarity) Actually this is a part of the cubic lattice remember lattices are infinite!
a = b = c,
o = | = = 90
A General Lattice in 3D
6 lattice parameters
3 distances (a, b, c)
3 angles (o, |, )
In special cases some of these numbers may
be equal to each other (e.g. a = b) or equal to
a special number (e.g. | = 90)
(hence we may not require 6 independent numbers to describe a lattice)
Any general
parallelepiped is space
filling
a = b = c, o = | =
Click here to
know more
about
A lattice is a set of points constructed by translating a single point in
discrete steps by a set of basis vectors.
In three dimensions, there are 14 unique Bravais lattices (distinct from one
another in that they have different space groups) in three dimensions. All
crystalline materials recognized till now fit in one of these arrangements.
In geometry and crystallography, a Bravais lattice is an infinite set of points
generated by a set of discrete translation operations.
A Bravais lattice looks exactly the same no matter from which point in the lattice
one views it.
Bravais concluded that there are only 14 possible Space Lattices (with Unit Cells
to represent them). These belong to 7 Crystal systems.
There are 14 Bravais Lattices which are the Space Group symmetries of lattices
A derivation of the 14 Bravais lattices or the existence of 7 crystal systems will not be shown in this introductory course
Bravais Lattice: various viewpoints
An important property of a lattice
Time to fasten
you seat-belts the
next few slides
will take you on
a 10 g-force dive
IMPORTANT
Crystals and Crystal Systems are defined
based on Symmetry
& NOT
Based on the Geometry of the Unit Cell
Cubic Crystal
Does NOT imply a = b = c & o = | =
It implies the existence of two 3-fold axis in the structure
Intrigued!
Want to Know
More?
Example
IMPORTANT
If lattices are based on just translation
(Translational Symmetry (t))
then how come other Symmetries (especially
rotational) come into the picture while choosing the
Crystal System & Unit Cell for a lattice?
Why do we say that End Centred Cubic Lattice does not exist?
Isnt it sufficient that a = b = c & o = | = to call something cubic?
(why do we put End Centred Cubic in Simple Tetragonal?)
The issue comes because we want to put 14 Bravais lattices into 7 boxes (the 7 Crystal
Systems; the Bravais lattices have 7 distinct symmetries) and further assign Unit Cells
to them
The Crystal Systems are defined based on Symmetries (Rotational, Mirror, Inversion
etc. forming the Point Groups) and NOT on the geometry of the Unit Cell
The Choice of Unit Cell is based on Symmetry & Size (& Convention)
(in practice the choice of unit cell is left to us! but what we call the crystal is not!!)
Continued
ONCE MORE:
When we say End Centred Cubic
End Centred is a type of Lattice (based on translation)
&
Cubic is a type of Crystal (based on other symmetries)
&
Cubic also refers to a shape of Unit Cell (based on lattice parameters)
AND:
To confuse things further
Cubic crystals can have lower symmetry than the cubic lattice
(e.g. Cubic lattices always have 4-fold axis while Cubic Crystals may not have 4-fold axes)
hang on! some up-coming examples will make things CRYSTAL clear
Feeling lost!?!
To emphasize:
The word Cubic (e.g. in a cubic crystal) refers to 3 things
A type of Lattice (based on translation)
&
A type of Crystal (based on other symmetries)
&
A shape of Unit Cell (based on lattice parameters)
Hence the confusion!!
Lattices have the highest symmetry
(Which is allowed for it)
Crystals based on the lattice
can have lower symmetry
Another
IMPORTANT point
Click here to know more
Crystal System Shape of UC Bravais Lattices
P I F C
1 Cubic Cube
2 Tetragonal Square Prism (general height)
3 Orthorhombic Rectangular Prism (general height)
4 Hexagonal 120 Rhombic Prism
5 Trigonal Parallopiped (Equilateral, Equiangular)
6 Monoclinic Parallogramic Prism
7 Triclinic Parallelepiped (general)
Why are some of the entries missing?
Why is there no C-centred cubic lattice?
Why is the F-centred tetagonal lattice missing?
.?
14 Bravais Lattices divided into 7 Crystal Systems
Continued
P Primitive
I Body Centred
F Face Centred
C A/B/C- Centred
A Symmetry based concept
We will take up these cases one by one
(hence do not worry!)
Translation based concept
Some guidelines apply
Arrangement of lattice points in the Unit Cell
& No. of Lattice points / Cell
Position of lattice points Effective number of Lattice points / cell
1 P 8 Corners = [8 (1/8)] = 1
2 I
8 Corners
+
1 body centre
= [1 (for corners)] + [1 (BC)] = 2
3 F
8 Corners
+
6 face centres
= [1 (for corners)] + [6 (1/2)] = 4
4
A/
B/
C
8 corners
+
2 centres of opposite faces
= [1 (for corners)] + [2 (1/2)] = 2
1 Cubic Cube
P I F C
Lattice point
P
I
F
a b c = =
90 o | = = =
4 2
3
m m
Symmetry of Cubic lattices
P I F C
2 Tetragonal Square Prism (general height)
I
P
a b c = =
90 o | = = =
Symmetry of Tetragonal lattices
4 2 2
m m m
P I F C
3 Orthorhombic Rectangular Prism (general height)
P
I
F
C
a b c = =
90 o | = = =
Symmetry of Orthorhombic lattices
2 2 2
m m m
Note the position of
a and b
a b c < <
One convention
Why is Orthorhombic called Ortho-Rhombic? Is there a alternate possible set of unit cells for OR?
P I F C
4 Hexagonal 120 Rhombic Prism
What about the HCP?
(Does it not have an additional atom somewhere in the middle?)
A single unit cell (marked in blue)
along with a 3-unit cells forming a
hexagonal prism
Note: there is only one type of hexagonal
lattice (the primitive one)
a b c = =
90 , 120 o | = = =
Symmetry of Hexagonal lattices
6 2 2
m m m
P I F C
5 Trigonal Parallelepiped (Equilateral, Equiangular)
90 o | = = =
a b c = =
Symmetry of Trigonal lattices
Rhombohedral
2
3
m
Note the position of the origin
and of a, b & c
Some times an alternate hexagonal cell
is used instead of the Trigonal Cell
A trigonal cell can be produced from a cubic
cell by pulling along [111] (the body diagonal)
(keeping the edge length of the cube constant)
P I F C
6 Monoclinic Parallogramic Prism
90 o | = = =
a b c = =
Symmetry of Monoclinic lattices
2
m
a b c < <
Note the position of
a, b & c
One convention
P I F C
7 Triclinic Parallelepiped (general)
a b c = =
o | = =
Symmetry of Triclinic lattices
1
Let us make some 3-D crystals Click here
An important property of a lattices
If one sits at any lattice point the space around looks identical to the person
This aspect might seem trivial here but is very useful to remember!
Hence we can chart out a set of equivalent points in space
(Which may or may not coincide with the lattice points)
1D
The Xs themselves form an equivalent lattice
2D
3D
Hence, if for a given crystal (say with FCC lattice decorated with a single atom motif), the edge centre is a position of an
octahedral void then the set of octahedral void positions will form a FCC lattice
Solved
Example
The Graphene Crystal
Q: I have seen a different representation of the
same unit cell WITHOUT the 6-folds. How come?
As we know lattices have the highest symmetry and hence a 120 rhombus lattice (noting
that this is actually the shape of the UC) always has 6-fold symmetries
However crystals based on the lattice can have lower symmetry which includes only 3-
fold symmetries
The list of crystals in 2D are (with shapes of UC):
Square Rectangle 120 Rhombus Parallelogram (general)
Unfortunately this does not include a crystal with 3-fold symmetry alone (which could be
called TRIANGULAR analogous to Trigonal in 3D)
Crystal Symmetries of the Crystal
Hence the 120 Rhombus lattice always has 6-fold axes while crystals based on the lattice may have only 3-folds
Note the loss
in a mirror as
well
Back
Click here Example of a 3D analogue of this
Das könnte Ihnen auch gefallen
- Aluminum Design Manual 2015Dokument505 SeitenAluminum Design Manual 2015Charlie Henke100% (2)
- (Lab Report Operation Unit) Experiment 3: Separation of An Ordinary Binary Mixture Consisting of Acetic Acid and Water by Using Simple Batch Distillation Technique.Dokument8 Seiten(Lab Report Operation Unit) Experiment 3: Separation of An Ordinary Binary Mixture Consisting of Acetic Acid and Water by Using Simple Batch Distillation Technique.Fazsroul100% (9)
- TEST REPORT After Earth ImprovementDokument3 SeitenTEST REPORT After Earth ImprovementShami Mudunkotuwa78% (9)
- Https - Myguru - Upsi.edu - My - Documents - 2019 - Courses - SFT3053 - Material - K00926 - 20190912094449 - Chapter 1 FLS PDFDokument52 SeitenHttps - Myguru - Upsi.edu - My - Documents - 2019 - Courses - SFT3053 - Material - K00926 - 20190912094449 - Chapter 1 FLS PDFNurazin RizalNoch keine Bewertungen
- HW1 PDFDokument8 SeitenHW1 PDFxabihdez05Noch keine Bewertungen
- Polypac BalseleDokument18 SeitenPolypac BalseleRenato GouveiaNoch keine Bewertungen
- LatticeDokument56 SeitenLatticeCarlos UrregoNoch keine Bewertungen
- LatticeDokument56 SeitenLatticeashok pradhanNoch keine Bewertungen
- LatticeDokument59 SeitenLatticeashishkr23438Noch keine Bewertungen
- Condensed Matter Physics Unit 1Dokument112 SeitenCondensed Matter Physics Unit 1anujjuetNoch keine Bewertungen
- HW1Dokument8 SeitenHW1Kevin Johnmar Urcia VidarteNoch keine Bewertungen
- CH 3 RaghavanDokument17 SeitenCH 3 Raghavantera baapNoch keine Bewertungen
- Solid State Theory - EDokument27 SeitenSolid State Theory - Ethinkiit100% (3)
- Circles Cheat Sheet DTPDokument5 SeitenCircles Cheat Sheet DTPJohnNoch keine Bewertungen
- Symmetry: Translations (Lattices)Dokument27 SeitenSymmetry: Translations (Lattices)CJPATAGANNoch keine Bewertungen
- Laue & Bravis Crystal LatticeDokument18 SeitenLaue & Bravis Crystal LatticeRSLNoch keine Bewertungen
- Mineralogy12 Lecture 2Dokument30 SeitenMineralogy12 Lecture 2Anonymous 6iZPHgIKOQNoch keine Bewertungen
- Assignment 1Dokument3 SeitenAssignment 1KuthuraikaranNoch keine Bewertungen
- CrystalDokument56 SeitenCrystalPrinceNoch keine Bewertungen
- Sem 1 Unit 3 Crystal Structure Final NotesDokument63 SeitenSem 1 Unit 3 Crystal Structure Final Notespwasu12350Noch keine Bewertungen
- Geometry Worksheet 1 - AnglesDokument13 SeitenGeometry Worksheet 1 - AnglesAlexaNoch keine Bewertungen
- 4 - Crystal Structure PDFDokument66 Seiten4 - Crystal Structure PDFManoj SelvamNoch keine Bewertungen
- Crystal Geometry 6upDokument11 SeitenCrystal Geometry 6upTan Phung NguyenNoch keine Bewertungen
- MO 201: Materials Science: DR Bratindranath Mukherjee Dept. of Metallurgical Engineering Bratindra - Met@itbhu - Ac.inDokument109 SeitenMO 201: Materials Science: DR Bratindranath Mukherjee Dept. of Metallurgical Engineering Bratindra - Met@itbhu - Ac.inRashmiNoch keine Bewertungen
- AreasDokument16 SeitenAreasShaik BajiNoch keine Bewertungen
- 7.1: Crystal Structure: CrystallographyDokument11 Seiten7.1: Crystal Structure: CrystallographyShofiNoch keine Bewertungen
- Angles Properties in CirclesDokument13 SeitenAngles Properties in Circlesjavaria227204Noch keine Bewertungen
- Classification of Lattices and StructuresDokument6 SeitenClassification of Lattices and StructuresBilal BarutNoch keine Bewertungen
- OpenSCAD User Manual-LanguageDokument38 SeitenOpenSCAD User Manual-Languagealarue100% (1)
- Solid State Physics IntroDokument25 SeitenSolid State Physics IntroJonatan Vignatti MuñozNoch keine Bewertungen
- Video Lecture For MBADokument135 SeitenVideo Lecture For MBAedholecomNoch keine Bewertungen
- 1 EBSD PrincipleDokument49 Seiten1 EBSD PrincipleandrianidebrinaNoch keine Bewertungen
- Crystal Geometry and Structure DeterminationDokument36 SeitenCrystal Geometry and Structure DeterminationABHINAV KUMAR SHUKLANoch keine Bewertungen
- Polarization Controller Stanford LefevreDokument5 SeitenPolarization Controller Stanford Lefevreapi-27533561Noch keine Bewertungen
- Crystallography and Structure of Elements: LatticesDokument33 SeitenCrystallography and Structure of Elements: LatticesVictor ParraNoch keine Bewertungen
- Making A 3D Crystal: There Are 14 Distinct Lattices Possible in 3D Called TheDokument20 SeitenMaking A 3D Crystal: There Are 14 Distinct Lattices Possible in 3D Called ThenewteamNoch keine Bewertungen
- M44 Polar Circles - EkPolarCoordDokument6 SeitenM44 Polar Circles - EkPolarCoordNoraNoch keine Bewertungen
- Ch1introduction Solid State PhysicsDokument34 SeitenCh1introduction Solid State PhysicsSohair SahedNoch keine Bewertungen
- Introduction Crystal PhysicsDokument118 SeitenIntroduction Crystal PhysicsSilambarasan Physics60% (5)
- X-Ray Diffraction: Strcuture of MaterialsDokument55 SeitenX-Ray Diffraction: Strcuture of MaterialsAahil AleemNoch keine Bewertungen
- Topic 03Dokument19 SeitenTopic 03Mohammed AbdoNoch keine Bewertungen
- C 1 Quadratic InterpolationDokument18 SeitenC 1 Quadratic InterpolationSukuje Je100% (1)
- Simple Crystal Structures-2 PDFDokument135 SeitenSimple Crystal Structures-2 PDFRoslina ShariffNoch keine Bewertungen
- Algebraic FormulaDokument73 SeitenAlgebraic Formulagspkishore7953Noch keine Bewertungen
- Teori Grup Dan Vibrasi-RevisiDokument204 SeitenTeori Grup Dan Vibrasi-RevisiDhieka NopiharguNoch keine Bewertungen
- Lecture 01 ADokument39 SeitenLecture 01 Akaran sahniNoch keine Bewertungen
- CH 4 NotesDokument15 SeitenCH 4 NotesMarina XuNoch keine Bewertungen
- Fisika Zat Padat I: Dosen: Dr. Iwantono, M.Phil Jurusan Fisika Fmipa-UrDokument78 SeitenFisika Zat Padat I: Dosen: Dr. Iwantono, M.Phil Jurusan Fisika Fmipa-UrMailestari Wina YanceNoch keine Bewertungen
- Monday PatelDokument38 SeitenMonday PatelSam AhmedNoch keine Bewertungen
- 07 GemoetryDokument11 Seiten07 GemoetryKomanduri Murali SrinivasNoch keine Bewertungen
- Crystal StructureDokument123 SeitenCrystal StructuresupyyyyNoch keine Bewertungen
- Introduction 2Dokument30 SeitenIntroduction 2niteshNoch keine Bewertungen
- Math Foundation Review Teacher's Edition: TrigonometryDokument24 SeitenMath Foundation Review Teacher's Edition: TrigonometrytwsttwstNoch keine Bewertungen
- Geogebra: X BX+C 1 B C (0,1) (B, 0) (0, C)Dokument10 SeitenGeogebra: X BX+C 1 B C (0,1) (B, 0) (0, C)Pearl RosarioNoch keine Bewertungen
- WK 5&6 - Secants, Tangents, Segments, and Sectors of A CircleDokument42 SeitenWK 5&6 - Secants, Tangents, Segments, and Sectors of A CircleJENNALYN RESARE100% (1)
- Cse169 04Dokument57 SeitenCse169 04Ibrahim Nazir100% (1)
- Lec 22Dokument12 SeitenLec 22stathiss11Noch keine Bewertungen
- Robot Manipulators: Modeling, Performance Analysis and ControlVon EverandRobot Manipulators: Modeling, Performance Analysis and ControlNoch keine Bewertungen
- The Scaled Boundary Finite Element Method: Introduction to Theory and ImplementationVon EverandThe Scaled Boundary Finite Element Method: Introduction to Theory and ImplementationNoch keine Bewertungen
- Wheelchair Lift Executive SummaryDokument5 SeitenWheelchair Lift Executive SummaryIsabelle HanafiahNoch keine Bewertungen
- Repair of Small Household Appliances and Power ToolsDokument315 SeitenRepair of Small Household Appliances and Power ToolsahmadnawazjaswalNoch keine Bewertungen
- Gasket ParametersDokument9 SeitenGasket Parametersttr_1947Noch keine Bewertungen
- Gapura Company Profile - 17mar17Dokument43 SeitenGapura Company Profile - 17mar17als izmiNoch keine Bewertungen
- Free Body DiagramDokument8 SeitenFree Body Diagramabdullah hamba allahNoch keine Bewertungen
- NAME Dendi Panji Nugraha: Career ExperiencesDokument4 SeitenNAME Dendi Panji Nugraha: Career ExperiencesAsep Ricky HerdiansyahNoch keine Bewertungen
- Interurban RMC Rail Bus ManualDokument4 SeitenInterurban RMC Rail Bus ManualSteven GardnerNoch keine Bewertungen
- Data Envelopment Analysis: Models and Extensions: Production/Operations ManagementDokument4 SeitenData Envelopment Analysis: Models and Extensions: Production/Operations Managementmithunrecdgp100% (2)
- How To Build Your Own Solid State OscilloscopeDokument100 SeitenHow To Build Your Own Solid State OscilloscopemariopilarNoch keine Bewertungen
- Xdo XliffloaderDokument7 SeitenXdo XliffloaderRenuka ChavanNoch keine Bewertungen
- The Centaur Upper Stage Vehicle HistoryDokument12 SeitenThe Centaur Upper Stage Vehicle HistoryjuniormirandaNoch keine Bewertungen
- Crane Operator Rigger TrainingDokument55 SeitenCrane Operator Rigger TrainingAli Jalil80% (5)
- Formula Notes For Cracking Fluid Mechanics For Gate by Jain SirDokument27 SeitenFormula Notes For Cracking Fluid Mechanics For Gate by Jain SirSachin Saini93% (27)
- Safety Incident ReportDokument10 SeitenSafety Incident ReportMelanie BrittainNoch keine Bewertungen
- Thermozorb Heatless Regenerative Air Dryer: TZ22 - TZ142Dokument2 SeitenThermozorb Heatless Regenerative Air Dryer: TZ22 - TZ142Емил ГавриловNoch keine Bewertungen
- Ahmad Mustaqiem CVDokument4 SeitenAhmad Mustaqiem CVAhmad MustaqiemNoch keine Bewertungen
- Bulk CollectDokument5 SeitenBulk CollectAbhishekNoch keine Bewertungen
- Under Balanced OperationsDokument104 SeitenUnder Balanced OperationsJavier Ignacio Meriles100% (1)
- Review On Development of Polypropylene Manufacturing ProcessDokument11 SeitenReview On Development of Polypropylene Manufacturing ProcessShweta Yadav100% (1)
- Final Fine Black Sport 06.12.23Dokument15 SeitenFinal Fine Black Sport 06.12.23NATWAR PRAJAPATINoch keine Bewertungen
- Cama Stryker Sv2-Service ManualDokument117 SeitenCama Stryker Sv2-Service ManualjoelpalzaNoch keine Bewertungen
- Practical Application of Pervious Concrete - Mix Designs That Are WorkableDokument20 SeitenPractical Application of Pervious Concrete - Mix Designs That Are WorkablePJ FlexirNoch keine Bewertungen
- CPP ReportDokument5 SeitenCPP ReportSujay Hazra100% (1)
- Poster Flender Com1 PDFDokument1 SeitePoster Flender Com1 PDFvijaykumarnNoch keine Bewertungen
- Systemd CheatsheetDokument1 SeiteSystemd Cheatsheetzaffa11Noch keine Bewertungen