Beruflich Dokumente
Kultur Dokumente
Heat 4.1 Understanding Thermal Equilibrium
Hochgeladen von
Az MYOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Heat 4.1 Understanding Thermal Equilibrium
Hochgeladen von
Az MYCopyright:
Verfügbare Formate
HEAT 4.
1 UNDERSTANDING THERMAL EQUILIBRIUM
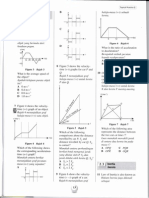
Define: (a) Temperature The measure of the degree of hotness of an object. Measured in SI unit Kelvin, K A hot object is at a higher temperature than a cold object. (b) Heat Form of energy, measured in Joules, J Heat is transferred from hotter object (higher temperature) to colder object (lower temperature) When an object is heated, it will absorb heat energy and the temperature will increase. When an object is cooled, it will release heat energy and the temperature will decrease. (c) Thermal contact Two objects are in thermal contact when heat energy can be transferred between them. (d) Heat transfer When two objects with different degrees of hotness come into thermal contact, heat energy is transferred between the two objects.
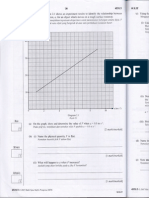
Liquid-in-glass Thermometer The characteristic of the liquid used in liquid-in-glass thermometer 1. be easily seen 2. expand and contract rapidly over a wide range of temperature/ expand uniformly when heated 3. not stick to the glass wall of the capillary tube. How a liquid-in-glass thermometer works? 1. The bulb of the thermometer contains a fixed mass of mercury. The volume of the mercury increases when it absorbs heat. 2. The mercury expands and rises in the capillary tube. The length of the mercury column in the capillary tube indicates the magnitude of the temperature.
4.2 UNDERSTANDING SPECIFIC HEAT CAPACITY 1. Heat capacity, C - The amount of heat required to change its temperature by one degree. 2. Specific Heat capacity, c The amount of heat that must be supplied to increase the temperature by 1 C for a mass of 1 kg of the substance Specific heat capacity, c = Q SI unit: = J kg-1C-1 m Q = heat absorbed / released, unit J m = mass of the substance, unit kg = temperature difference , unit C
Applications of Specific Heat Capacity Cooking pot (a) Copper base (b) Wooden Handle
(c) Alumni body Low specific heat capacity. The pot becomes hot very quickly. This enables quick cooking of the food in the pot. High density. The heavier base ensures that the pot is stable and will not topple over easily. Large specific heat capacity. The handle will not become too hot when heat is absorbed. Poor conductor of heat. Relatively low specific heat capacity. The pot becomes hot quickly. Low density so it will be lighter Does not react with the food in the pot
Sea Breeze Land has a smaller specific heat capacity than sea. Faster increase in temperature, ie land is warmer than the sea Air above the land is heated up and rises. Cooler air from the sea moves from towards the land as sea breeze.
Specific Latent Heat, l The amount of heat required to change the phase of 1 kg of the substance at a constant temperature. ml = Q 69 unit : J kg-1 Q = latent heat absorbed or released by the substance m = mass of the substance Specific latent heat of fusion The amount of heat required to change 1 kg of the substance from solid to liquid phase without a change in temperature. Specific latent heat of vaporization The amount of heat required to change 1 kg of the substance from the liquid to gaseous phase without a change in temperature.
Das könnte Ihnen auch gefallen
- Hot Globe and No Greenery Courtesy of the Carbon Hater's CultVon EverandHot Globe and No Greenery Courtesy of the Carbon Hater's CultNoch keine Bewertungen
- Nota Padat Fizik F4 Heat NotesDokument17 SeitenNota Padat Fizik F4 Heat Notesslokkro99% (109)
- Carmenphysicsheat 160713161011 PDFDokument76 SeitenCarmenphysicsheat 160713161011 PDFDa DnyalNoch keine Bewertungen
- The Measure of The Degree of Hotness of An ObjectDokument17 SeitenThe Measure of The Degree of Hotness of An Objectshuk_edu_896744Noch keine Bewertungen
- Nota Padat Fizik F4 Heat Notes SignedDokument17 SeitenNota Padat Fizik F4 Heat Notes SignedkwNoch keine Bewertungen
- Form 4 Chapter 4: Heat: Understanding Thermal EquilibriumDokument10 SeitenForm 4 Chapter 4: Heat: Understanding Thermal EquilibriumbatrisyiaNoch keine Bewertungen
- Thermal Equilibrium: TemperatureDokument21 SeitenThermal Equilibrium: TemperatureFadlan RoslanNoch keine Bewertungen
- Bab 3 Heat (ICO7)Dokument4 SeitenBab 3 Heat (ICO7)anung edy nugrohoNoch keine Bewertungen
- Haba Dan TenagaDokument20 SeitenHaba Dan TenagahisharuddinNoch keine Bewertungen
- Form 4 Physics - HeatDokument8 SeitenForm 4 Physics - HeatDenisse ChiaNoch keine Bewertungen
- Heat (Add Science) OkDokument35 SeitenHeat (Add Science) OkJaswardi Anwar Bin Md Yaacob� IPGKKBNoch keine Bewertungen
- Heat NotesDokument105 SeitenHeat NotesNuan Ting NgNoch keine Bewertungen
- 4.2 Specific Heat Cap 2020Dokument51 Seiten4.2 Specific Heat Cap 2020zaim aqeelNoch keine Bewertungen
- Chapter 4.2Dokument5 SeitenChapter 4.2Siah Woan ChiouNoch keine Bewertungen
- HeatDokument33 SeitenHeatVyvian LeowNoch keine Bewertungen
- Chap 4 SummaryDokument6 SeitenChap 4 SummaryAzaizulAhmadNoch keine Bewertungen
- Specific Heat Capacity - MahaDokument33 SeitenSpecific Heat Capacity - MahaMaha Letchumy BalakeristananNoch keine Bewertungen
- Chapter 10Dokument44 SeitenChapter 10Jayaletchumi T. Sambantha MoorthyNoch keine Bewertungen
- 4.1 Understanding Thermal Equilibrium: Thermometric PropertiesDokument24 Seiten4.1 Understanding Thermal Equilibrium: Thermometric PropertiesSyamimi Abd RahmanNoch keine Bewertungen
- 4.2 Physics Form 4Dokument29 Seiten4.2 Physics Form 4871226Noch keine Bewertungen
- UntitledDokument38 SeitenUntitledmuhammad sallehuddin ismailNoch keine Bewertungen
- 4.1 Understanding Thermal Equilibrium: Chapter Summary / Chap. 4/ HeatDokument6 Seiten4.1 Understanding Thermal Equilibrium: Chapter Summary / Chap. 4/ HeatkhodijahaminNoch keine Bewertungen
- 12 141019201725 Conversion Gate02 PDFDokument117 Seiten12 141019201725 Conversion Gate02 PDFMohammed AmliehNoch keine Bewertungen
- TempheatDokument28 SeitenTempheatapi-343650677Noch keine Bewertungen
- Specific HeatDokument16 SeitenSpecific HeatshaiwraaaNoch keine Bewertungen
- Thermal Physics - 1Dokument54 SeitenThermal Physics - 1big chungusNoch keine Bewertungen
- Thermal Energy and HeatDokument34 SeitenThermal Energy and HeatMarie Ann SeraficaNoch keine Bewertungen
- 12-Thermal Properties of Matter-OrigDokument117 Seiten12-Thermal Properties of Matter-Origrodel.verzosaNoch keine Bewertungen
- Calorimetry SynopsisDokument4 SeitenCalorimetry Synopsissreevaishnava01Noch keine Bewertungen
- HAAVINESH A - L GANESH Moe - THEME 3 HEAT 4.2 SPECIFIC HEAT CAPACITY - STUDENTDokument33 SeitenHAAVINESH A - L GANESH Moe - THEME 3 HEAT 4.2 SPECIFIC HEAT CAPACITY - STUDENTHaavinesh Ganesh100% (1)
- Suhu Dan KalorDokument31 SeitenSuhu Dan KalorNella Sri PujirahayuNoch keine Bewertungen
- TP5 - Heat Capacity & Latent Heat 1Dokument5 SeitenTP5 - Heat Capacity & Latent Heat 1wengiemotshegweNoch keine Bewertungen
- Thermal PhysicsDokument45 SeitenThermal PhysicsChristine MalibiranNoch keine Bewertungen
- Chapter 2 IGCSE - ActualDokument7 SeitenChapter 2 IGCSE - ActualNajia UmarNoch keine Bewertungen
- How Hurricanes Pick Up EnergyDokument23 SeitenHow Hurricanes Pick Up EnergyAhanaNoch keine Bewertungen
- Thermal Properties CSEC Physics 2020Dokument34 SeitenThermal Properties CSEC Physics 2020Mariah CampbellNoch keine Bewertungen
- Xi Physics - Thermal Properties of MatterDokument14 SeitenXi Physics - Thermal Properties of MatteradarshdarasinghNoch keine Bewertungen
- 4.2 Specific Heat CapacityDokument19 Seiten4.2 Specific Heat CapacitykhodijahaminNoch keine Bewertungen
- Kelompok 2 Kalor Dan Perubahan KalorDokument37 SeitenKelompok 2 Kalor Dan Perubahan KalorNurul JasminNoch keine Bewertungen
- Module Heat - Answer SchemeDokument27 SeitenModule Heat - Answer SchemeCart KartikaNoch keine Bewertungen
- Heat QuantitiyDokument32 SeitenHeat QuantitiyMagued MikhaelNoch keine Bewertungen
- Practice Makes Perfect in Chemistry: The Physical Behavior of MatterVon EverandPractice Makes Perfect in Chemistry: The Physical Behavior of MatterBewertung: 5 von 5 Sternen5/5 (1)
- On Specific Heat, Pyrolysis, Transponders, Et AliiVon EverandOn Specific Heat, Pyrolysis, Transponders, Et AliiNoch keine Bewertungen
- Practice Makes Perfect in Chemistry: The Physical Behavior of Matter with AnswersVon EverandPractice Makes Perfect in Chemistry: The Physical Behavior of Matter with AnswersNoch keine Bewertungen
- The Energy That Warms Us: A Look at HeatVon EverandThe Energy That Warms Us: A Look at HeatBewertung: 1 von 5 Sternen1/5 (1)
- Scientific American Supplement, No. 455, September 20, 1884Von EverandScientific American Supplement, No. 455, September 20, 1884Noch keine Bewertungen
- You Can Prevent Global Warming (and Save Money!): 51 Easy WaysVon EverandYou Can Prevent Global Warming (and Save Money!): 51 Easy WaysBewertung: 4 von 5 Sternen4/5 (40)
- Liquid Helium Technology: Proceedings of the International Institute of Refrigeration Commission 1, Boulder (U.S.A.) 1966Von EverandLiquid Helium Technology: Proceedings of the International Institute of Refrigeration Commission 1, Boulder (U.S.A.) 1966Noch keine Bewertungen
- 50 Climate Questions: A Blizzard of Blistering FactsVon Everand50 Climate Questions: A Blizzard of Blistering FactsBewertung: 3 von 5 Sternen3/5 (1)
- Mechanics of the Household: A Course of Study Devoted to Domestic Machinery and Household Mechanical AppliancesVon EverandMechanics of the Household: A Course of Study Devoted to Domestic Machinery and Household Mechanical AppliancesNoch keine Bewertungen
- Global Warming: 50 tips about the hot topic many people dismissVon EverandGlobal Warming: 50 tips about the hot topic many people dismissNoch keine Bewertungen
- Oceans, Glaciers, and Rising Sea Levels: A Graphic GuideVon EverandOceans, Glaciers, and Rising Sea Levels: A Graphic GuideNoch keine Bewertungen
- The Big Book of Science: The Ultimate Children's GuideVon EverandThe Big Book of Science: The Ultimate Children's GuideNoch keine Bewertungen
- Alternating Current CircuitsDokument2 SeitenAlternating Current CircuitsAz MYNoch keine Bewertungen
- 10.1 Heat CapacitiesDokument2 Seiten10.1 Heat CapacitiesAz MYNoch keine Bewertungen
- Physics STPMDokument9 SeitenPhysics STPMAz MYNoch keine Bewertungen
- Checkpoint 10.1Dokument1 SeiteCheckpoint 10.1Az MYNoch keine Bewertungen
- Checkpoint 9.4Dokument1 SeiteCheckpoint 9.4Az MYNoch keine Bewertungen
- 10.1 Heat CapacitiesDokument2 Seiten10.1 Heat CapacitiesAz MYNoch keine Bewertungen
- Checkpoint 11.1Dokument2 SeitenCheckpoint 11.1Az MYNoch keine Bewertungen
- Read and Understand The Notes. Answer The Examples Questions BelowDokument1 SeiteRead and Understand The Notes. Answer The Examples Questions BelowAz MYNoch keine Bewertungen
- Checkpoint 11.1Dokument2 SeitenCheckpoint 11.1Az MY100% (1)
- Read and Understand The Notes. Answer The Examples Questions BelowDokument1 SeiteRead and Understand The Notes. Answer The Examples Questions BelowAz MYNoch keine Bewertungen
- Checkpoint 10.1Dokument1 SeiteCheckpoint 10.1Az MYNoch keine Bewertungen
- Checkpoint 9.4Dokument1 SeiteCheckpoint 9.4Az MYNoch keine Bewertungen
- Img 20140413 0001Dokument1 SeiteImg 20140413 0001Az MYNoch keine Bewertungen
- Paper 3Dokument16 SeitenPaper 3Az MYNoch keine Bewertungen
- 18.3 Alternating Current Through A Capacitor PDFDokument6 Seiten18.3 Alternating Current Through A Capacitor PDFAz MY100% (1)
- 18.4 RC and RL Circuits in Series PDFDokument5 Seiten18.4 RC and RL Circuits in Series PDFAz MYNoch keine Bewertungen
- 18.2 Alternating Currect Through An Inductor PDFDokument9 Seiten18.2 Alternating Currect Through An Inductor PDFAz MYNoch keine Bewertungen
- Peperiksaan Percubaan Sijil Pelajaran Malaysia 2008: FizikDokument7 SeitenPeperiksaan Percubaan Sijil Pelajaran Malaysia 2008: FizikAz MYNoch keine Bewertungen
- InertiaDokument21 SeitenInertiaAz MY100% (1)
- Peta 1Dokument1 SeitePeta 1Az MYNoch keine Bewertungen
- Img 20140511 0007Dokument1 SeiteImg 20140511 0007Az MYNoch keine Bewertungen
- L!Tl1 Nldlaq Eqljo Eql ( (L//: ElelsDokument1 SeiteL!Tl1 Nldlaq Eqljo Eql ( (L//: ElelsAz MYNoch keine Bewertungen
- Img 20140306 0004Dokument1 SeiteImg 20140306 0004Az MYNoch keine Bewertungen
- Chapter 3 P2 IntensiveDokument23 SeitenChapter 3 P2 IntensiveAz MYNoch keine Bewertungen
- Img 20140310 0005Dokument1 SeiteImg 20140310 0005Az MYNoch keine Bewertungen