Beruflich Dokumente
Kultur Dokumente
Manage farm QMS docs
Hochgeladen von
muley_jayOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Manage farm QMS docs
Hochgeladen von
muley_jayCopyright:
Verfügbare Formate
QMS Documentation
Click the mouse to advance slides and animations in this slide show
QMS Manuals and Documentation
Your core QMS Documentation is intended to be stored, read, and edited on your computer because you may:
backup and protect your work/files on CD. prevent unauthorized access or editing of documents through file security options (i.e., read only or password protected documents). Ensure that the most current version of the documentation is available to your employees and customers, and that other obsolete documents are appropriately managed/controlled.
QMS Manuals and Documentation
The majority of your QMS documentation is retained in 2 manuals on your computer:
*Quality Manual *Procedures Manual
You should have already downloaded these by following Exercise 0.1 in your Workbook.
*Quality Manual
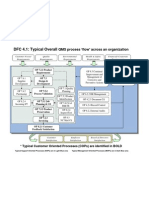
An introduction to your farm business, including the SCOPE of your QMS. A description of your farms processes and their interactions (i.e., PROCESS FLOW DIAGRAM) Your Farms commitment to quality management (i.e., QUALITY POLICY)
Allegiance to ISO 9001:2000 for your QMS. (i.e., SECTIONS 4-8 of the Quality Manual)
*Procedures Manual
The 6 PROCEDURES that are required by ISO 9001:2000
14-19 RECORDS that are required by ISO 9001:2000
Additional documentation that is needed to ensure effective planning, operation, and control of farm processes.
*Procedures Manual
1. Document Control 2. Record Control 3. Internal Audit
4. 5. 6.
Control of Non-Conforming Product Corrective Actions Preventative Actions
Some of these procedures have already been added to your Manual (e.g., #1,2). Example procedures are also provided in the Ag-ISO WebPages.
You will learn more about each of these while you work through the exercises.
*Procedures Manual
1. Management review records 2. Records of personnel education, training, skills, and experience 3. Records demonstrating that the products (and realization processes) meet all customer and legal requirements. 4. Records of the review of product requirements prior to realization 5. Supplier evaluation and selection records 6. Validation of process for production and service provision records 7. Product Identification and traceability records 8. Records of loss or damage to customer property 9. Calibration verification 10. Internal audit records 11. Records of product conformity to requirements and release to customer 12. Records of Nonconforming products 13. Corrective action records 14. Preventative action records
*Procedures Manual
1. The 6 procedures and 14-19 records that are required according to ISO 9001:2000
2. Additional documentation that is needed to ensure effective planning, operation, and control of farm processes
*Procedures Manual
that are needed to ensure effective planning, operation, and control of farm processes.
The addition of procedures and records beyond those that are required by ISO 9001:2000 is completely up to you. You will start your QMS by meeting the minimum documentation requirements (shown on previous slide). You will then decide how additional documentation may be used to improve your QMS on an as needed basis. The Ag-ISO Workbook and WebPages will lead you through the development of your manuals. Keep in mind that you MUST put into practice what you document in your QMS. Your documentation should be useful, but dont write yourself into a corner by being too detailed or specific.
QMS Document Organization
You should have already downloaded the Quality and Procedures Manual templates following Exercise 0.1 of the Ag-ISO Workbook.
The following slides illustrate one method of QMS document organization that may help you to effectively keep track of your files.
QMS Document Organization
One The QMS When In Some some core of documentation of you the cases, your of set download benefits your records up the QMS your data of the will this will QMS Documentation you Quality be be method stored you collect printed Manual may of in as hard organization either want is part and generated, copies to of the Procedures organize your Manuals, of is QMS forms that stored, your you will Manual or ordocumentation and/or in external be can separate referenced back-up templates referenced into electronic from in records your your a QMS the main manuals, (e.g., Ag-ISO files. files in folder. two checklists, in These WebPages, but one manuals stored step may receipts). by on be in save copying your separate organized them They computers the electronic with should into QMS a 2 name hard subfolders. also Documentation files drive. be appropriate (e.g., referenced yieldto folder maps). in your the to a farm or Organize Procedures disk (e.g., CD. these XYZ Manual Farms in the and QMS Quality organized Records Manual.doc) insubfolder. a file cabinet in the Manuals that is insubfolder. a safe location.
Local Disk C:\ QMS Documentation Manuals
*Quality Manual *Procedures Manual
QMS Records (electronic) reference
QMS Records (hard copies)
Das könnte Ihnen auch gefallen
- How to Establish a Document Control System for Compliance with ISO 9001:2015, ISO 13485:2016, and FDA Requirements: A Comprehensive Guide to Designing a Process-Based Document Control SystemVon EverandHow to Establish a Document Control System for Compliance with ISO 9001:2015, ISO 13485:2016, and FDA Requirements: A Comprehensive Guide to Designing a Process-Based Document Control SystemNoch keine Bewertungen
- ISO 9001 Ver 2015 QMS DocumentationDokument33 SeitenISO 9001 Ver 2015 QMS Documentationheheehehe100% (1)
- Generic QMS TemplateDokument62 SeitenGeneric QMS Templatemadodandembe100% (1)
- Iso 9001:2008 Quality Management System Implementation Plan: PlanningDokument1 SeiteIso 9001:2008 Quality Management System Implementation Plan: PlanningKhirullah Abdul HamidNoch keine Bewertungen
- ISO 9001:2015 Internal Audit Checklist: WWW - Iso9001help - Co.ukDokument2 SeitenISO 9001:2015 Internal Audit Checklist: WWW - Iso9001help - Co.ukSwetha Rao0% (1)
- Quality Manual: UncontrolledDokument23 SeitenQuality Manual: UncontrolledSiddhartha SrivastavaNoch keine Bewertungen
- Control Sample ISO 9000Dokument9 SeitenControl Sample ISO 9000spongemouse80% (5)
- Project Checklist of ISO 9001Dokument2 SeitenProject Checklist of ISO 9001Rajat Jain100% (1)
- Quality Audit Checklist FOR ISO 9001:2000Dokument10 SeitenQuality Audit Checklist FOR ISO 9001:2000MAdrianRumayarNoch keine Bewertungen
- ISO 9001 Evidence ChecklistDokument40 SeitenISO 9001 Evidence ChecklistsanrexiNoch keine Bewertungen
- QMSDokument1 SeiteQMSVirginia GrandoNoch keine Bewertungen
- QMS Process ManualDokument157 SeitenQMS Process Manualbg_phoenixNoch keine Bewertungen
- Iso 9001 Implementation PlanDokument8 SeitenIso 9001 Implementation PlanPaul Ryan100% (3)
- ISO 9001 Audit ChecklistDokument4 SeitenISO 9001 Audit ChecklistTanzila Siddiqui100% (3)
- ISO 9001 Quality Manual - Far West TechnologyDokument31 SeitenISO 9001 Quality Manual - Far West TechnologyQuản Trị Xứ MườngNoch keine Bewertungen
- Quality ManualDokument24 SeitenQuality Manualezzularab100% (1)
- ISO 9001:2015 Quality Manual 2nd Edition PreviewDokument12 SeitenISO 9001:2015 Quality Manual 2nd Edition PreviewCentauri Business Group Inc.100% (12)
- Document Control ProcessDokument5 SeitenDocument Control Processmypenta2008Noch keine Bewertungen
- ISO 9001-2015 Supplier Audit ChecklistDokument23 SeitenISO 9001-2015 Supplier Audit ChecklistFadhel Audia YusranNoch keine Bewertungen
- ISO 9001 Implementation ScheduleDokument2 SeitenISO 9001 Implementation ScheduleqsukNoch keine Bewertungen
- MDSAP Corrective Actions ProcedureDokument11 SeitenMDSAP Corrective Actions ProcedureNixNoch keine Bewertungen
- ISO 9001 Quality Manual TemplateDokument43 SeitenISO 9001 Quality Manual TemplateNC Rigor Luis92% (13)
- QMS Manual Sample For ISO 9001:2015Dokument41 SeitenQMS Manual Sample For ISO 9001:2015Syaiful Rasyidi Tamsir100% (3)
- Implementing QMSDokument94 SeitenImplementing QMSAji Ashiq100% (1)
- NCR Vs EvidenceDokument2 SeitenNCR Vs EvidenceMAT-LION100% (1)
- Procedures Manual - IsoDokument214 SeitenProcedures Manual - IsoMaricel Santos Ad100% (4)
- Quality Management System: Prepared By: Furqan Ansari (PG-18-04) Apurva Deshmukh (PG-18-13)Dokument15 SeitenQuality Management System: Prepared By: Furqan Ansari (PG-18-04) Apurva Deshmukh (PG-18-13)Furqan AnsariNoch keine Bewertungen
- Control of Documents ProcedureDokument5 SeitenControl of Documents Procedureaileen_macayanNoch keine Bewertungen
- ISO 9001-2015 CluasesDokument1 SeiteISO 9001-2015 Cluasesmanuprbr100% (10)
- QMS Manual ISO 9001:2015Dokument30 SeitenQMS Manual ISO 9001:2015vivekcp8750% (2)
- Iso 9000 2015Dokument15 SeitenIso 9000 2015Vasudevan GovindarajNoch keine Bewertungen
- 01 Documented Information 1Dokument11 Seiten01 Documented Information 1Noor Muddassir KhanNoch keine Bewertungen
- ISO 9001 Quality Management System DocumentsDokument33 SeitenISO 9001 Quality Management System DocumentsSanjay Rajpal100% (1)
- ISO 9001:2015 QMS Implementation Program (Presentation)Dokument16 SeitenISO 9001:2015 QMS Implementation Program (Presentation)Centauri Business Group Inc.96% (46)
- BQ-9000 Quality Management System. Producer RequirementsDokument31 SeitenBQ-9000 Quality Management System. Producer RequirementsMartin Alberto Savi GualcoNoch keine Bewertungen
- ISO 9001 Quality ManualDokument29 SeitenISO 9001 Quality Manualnatrix029100% (2)
- Supplier/subcontractor Quality RequirementsDokument68 SeitenSupplier/subcontractor Quality RequirementsSagar ShahNoch keine Bewertungen
- Process Audit ChecklistDokument17 SeitenProcess Audit ChecklistKeith Hoodless100% (1)
- Iso 9001 Activity Plan '05Dokument3 SeitenIso 9001 Activity Plan '05jvenrqzNoch keine Bewertungen
- NIST SP 800-171r1Dokument125 SeitenNIST SP 800-171r1Fabio MartinezNoch keine Bewertungen
- Iso 9001 2015 Quality Objectives and DefinitionsDokument20 SeitenIso 9001 2015 Quality Objectives and Definitionsvenkata chowdary83% (12)
- ISO 9001 2015 GAP Analysis ReviewDokument58 SeitenISO 9001 2015 GAP Analysis ReviewabcNoch keine Bewertungen
- Quality Manual TemplateDokument19 SeitenQuality Manual Templatetito1628100% (1)
- ISO 9001 Toolkit Contents GuideDokument3 SeitenISO 9001 Toolkit Contents GuideEl KhanNoch keine Bewertungen
- Iso 9001-2015 Gap AnalysisDokument30 SeitenIso 9001-2015 Gap Analysisjamilvora100% (4)
- ISO 9001 2015 and Risk AssesmentDokument33 SeitenISO 9001 2015 and Risk AssesmentAbdel Nasser Al-sheikh Yousef100% (22)
- Management Review Wasit-1Dokument7 SeitenManagement Review Wasit-1SANUNoch keine Bewertungen
- Quality Management - ISO 9001 - 2015 Mandatory Documented Information - Documents and RecordsDokument6 SeitenQuality Management - ISO 9001 - 2015 Mandatory Documented Information - Documents and RecordsSithanandan GanapathyNoch keine Bewertungen
- Quality Management System StandardsDokument25 SeitenQuality Management System Standardsrasimc9475100% (5)
- Procedure For Control of RecordsDokument3 SeitenProcedure For Control of Recordsmatrixmaze50% (2)
- QMS DocumentationDokument11 SeitenQMS Documentationaji ibrahimNoch keine Bewertungen
- Iso ProceduresDokument57 SeitenIso ProceduresShin Mey100% (4)
- ISO 9001-2008 Internal Audit ChecklistDokument43 SeitenISO 9001-2008 Internal Audit Checklistshinto LawrenceNoch keine Bewertungen
- ISO 9001-2015 Process Audit ChecklistDokument18 SeitenISO 9001-2015 Process Audit Checklistprincess cipriano100% (5)
- QMS MANUAL - QMS MNL 51 001 - R0 - ApprovedDokument52 SeitenQMS MANUAL - QMS MNL 51 001 - R0 - ApprovedOladunni Afolabi100% (3)
- What Is A WhyDokument3 SeitenWhat Is A Whymuley_jayNoch keine Bewertungen
- Abrasive Jet MachinngDokument18 SeitenAbrasive Jet MachinngJayesh MuleyNoch keine Bewertungen
- Permutations Xi Xii Study MaterialsDokument44 SeitenPermutations Xi Xii Study MaterialsKaran ChadhaNoch keine Bewertungen
- Chemistry QuestDokument184 SeitenChemistry QuestAshish Kumar100% (2)
- Chemistry QuestDokument184 SeitenChemistry QuestAshish Kumar100% (2)
- Complex NumbersDokument89 SeitenComplex Numberschammak100% (14)
- Report On The Logic GatesDokument33 SeitenReport On The Logic GatesSamyak Sau63% (27)
- KVPY Question Paper 2011 STREAM SADokument29 SeitenKVPY Question Paper 2011 STREAM SASteven KingNoch keine Bewertungen
- KVPY Paper Solution XI 04-11-12Dokument23 SeitenKVPY Paper Solution XI 04-11-12muley_jayNoch keine Bewertungen
- KVPY EXAMINATION 2010 MATHEMATICS QUESTIONSDokument40 SeitenKVPY EXAMINATION 2010 MATHEMATICS QUESTIONSSwapan Kumar MajumdarNoch keine Bewertungen
- Class XI Career Point KVPY Examination 2010 Math and Physics QuestionsDokument18 SeitenClass XI Career Point KVPY Examination 2010 Math and Physics Questionsdhruv001Noch keine Bewertungen
- 9 2 PDFDokument3 Seiten9 2 PDFPritesh ShahNoch keine Bewertungen
- Sterling International Consulting FZE Initiates ISO 9001 Consulting Project With Rollys Relocations LLC in U.A.E, Dubai.Dokument2 SeitenSterling International Consulting FZE Initiates ISO 9001 Consulting Project With Rollys Relocations LLC in U.A.E, Dubai.Kaushal SutariaNoch keine Bewertungen
- The Contribution of 5S Towards Total Quality Management in the Marine IndustryDokument131 SeitenThe Contribution of 5S Towards Total Quality Management in the Marine IndustryFaiz IshakNoch keine Bewertungen
- SN200Dokument111 SeitenSN200tajinder231280% (5)
- Project Quality PlanDokument33 SeitenProject Quality PlanCharles OukoNoch keine Bewertungen
- Assignment-4: Country Director ITC - Feb 14Dokument3 SeitenAssignment-4: Country Director ITC - Feb 14FaysalNoch keine Bewertungen
- Ashbee Industries Pvt. Ltd. Plant Facilities ReportDokument16 SeitenAshbee Industries Pvt. Ltd. Plant Facilities ReportrmdarisaNoch keine Bewertungen
- General Catalogue: Cod. 9910204 - 10/2015 Stampa: Euroteam (BS)Dokument1.222 SeitenGeneral Catalogue: Cod. 9910204 - 10/2015 Stampa: Euroteam (BS)MSc Kostic MilosNoch keine Bewertungen
- Redbook Vol1part1 PDFDokument789 SeitenRedbook Vol1part1 PDFYalem AlemayehuNoch keine Bewertungen
- CHAPTER 1.1 - 1.6 Introduction - SPS 170Dokument26 SeitenCHAPTER 1.1 - 1.6 Introduction - SPS 170Fareez HamidNoch keine Bewertungen
- The New ISO IEC 17025 2017Dokument9 SeitenThe New ISO IEC 17025 2017Omar SalasNoch keine Bewertungen
- CS2055 - Software Quality AssuranceDokument15 SeitenCS2055 - Software Quality AssuranceHaripriya SridharanNoch keine Bewertungen
- Nice Eng PDFDokument10 SeitenNice Eng PDFRadhitya WirawanNoch keine Bewertungen
- 6 Project Development and Modern Trends in Project Management PerceptionDokument11 Seiten6 Project Development and Modern Trends in Project Management Perceptionmraju143Noch keine Bewertungen
- Saras Report DharmveerDokument45 SeitenSaras Report DharmveerBikram SinghNoch keine Bewertungen
- 10.FITOK Company Brochure 160909Dokument20 Seiten10.FITOK Company Brochure 160909inglegs75Noch keine Bewertungen
- ISO 9001 Internal Audit Checklist GuidanceDokument8 SeitenISO 9001 Internal Audit Checklist GuidanceFauzul MutaqinNoch keine Bewertungen
- ISO 9001 guide for continual improvementDokument12 SeitenISO 9001 guide for continual improvementKalish kumarNoch keine Bewertungen
- ISO IEC 17020-GapAnalysisCrossReferenceDokument20 SeitenISO IEC 17020-GapAnalysisCrossReferenceshaggerukNoch keine Bewertungen
- Schedule Q Based QuestionsDokument11 SeitenSchedule Q Based Questionschandu666creator100% (7)
- Certification Specifications For Standard Changes & Standard Repairs (CS-STAN) - Phase 1Dokument58 SeitenCertification Specifications For Standard Changes & Standard Repairs (CS-STAN) - Phase 1BENoNoch keine Bewertungen
- Effectiveness of AIS Development Procedures in Meeting End-User Requirements: A Case Study of Afrosoft (PVT) LTDDokument6 SeitenEffectiveness of AIS Development Procedures in Meeting End-User Requirements: A Case Study of Afrosoft (PVT) LTDEl GwekwerereNoch keine Bewertungen
- GueorguievDokument8 SeitenGueorguievAdel ToumiNoch keine Bewertungen
- Nota Slide Bab 4Dokument45 SeitenNota Slide Bab 4君忠何Noch keine Bewertungen
- Title Pages QMSDokument61 SeitenTitle Pages QMSJohn JosephNoch keine Bewertungen
- P.A. College Mechanical Engineering Dept PO/PSO Gaps 2010-2016Dokument14 SeitenP.A. College Mechanical Engineering Dept PO/PSO Gaps 2010-2016VarunNoch keine Bewertungen
- MANAGEMENT OF CHANGE PROCEDURE NetcoreDokument12 SeitenMANAGEMENT OF CHANGE PROCEDURE NetcoreAniekan AkpaidiokNoch keine Bewertungen
- Fluke Calibration CertificatesDokument8 SeitenFluke Calibration Certificatesusebio64Noch keine Bewertungen
- Case Studies - ISO 9001-2015Dokument2 SeitenCase Studies - ISO 9001-2015Jun Ooi100% (20)
- SQM 16 & 2marks With AnsDokument21 SeitenSQM 16 & 2marks With AnsKalyan SundaramNoch keine Bewertungen
- Curriculum Vitae: Ranjan BesekarDokument4 SeitenCurriculum Vitae: Ranjan BesekarIqbalAsifNoch keine Bewertungen