Beruflich Dokumente
Kultur Dokumente
Ch1 - Introduction
Hochgeladen von
Diraf AlipOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Ch1 - Introduction
Hochgeladen von
Diraf AlipCopyright:
Verfügbare Formate
1
Aman Mohd Ihsan
03-55436268
T1-A16-4C
1.0 Introduction
2
COURSE INFO
Code : MEC551
Course : THERMAL ENGINEERING
Contact Hrs : 3 (L) & 1 (T) / weeks
Course Status : Core
3
Course Outcomes
Upon Completion of this course, students
should be able to :
CO1 Describe the principles of heat transfer
mechanisms, combustion, refrigeration and air
conditioning systems [PO1, LO1]{C2}.
CO2 Establish relationship between theoretical and
practical aspects of heat transfer application
[PO1, LO1]{C3}.
CO3 Analyse principles of energy mechanisms to
solve a wide range of thermal engineering
problems [PO3, LO3, SS1]{C4, P4}.
CO4 Develop solutions for mathematical models and
propose appropriate results for thermal
engineering applications. [PO3, LO3, SS1]{C5}.
CO5 Show concern on energy utilization and its
impact on the environment. [PO9, LO6, SS4]{A3}.
5
Assessment
Coursework 40%
Test 1 15%
Test 2 15%
Assignments 10%
Final Exam 60%
6
Course Outline
1. Introduction 3 hrs
2. Conduction 7 hrs
3. Convection 6 hrs
4. Heat Exchangers 6 hrs
5. Combustion 6 hrs
6. Refrigeration Cycles 7 hrs
7. Air-conditoning Processes 7 hrs.
TEST 1 (~ Week 6-7)
TEST 2 (~ Week 11-12)
7
Text book
Y.A. Cengel, Heat and
Mass Transfer: A
Practical Approach,
McGraw-Hill, 3rd Edition,
2007.
Y.A. Cengel and M.A.
Boles,Thermodynamics:
An Engineering
Approach, , McGraw-Hill,
6th Edition, 2007.
1.0 INTRODUCTION
1.1 Fundamental mechanism of Heat Transfer:
Conduction, Convection and Radiation.
1.2 Ozone Depleting Substances and Global
Warming Issues.
1.3 Renewable Energy Resources and
Technologies - Sustainable Energy
Management.
9
Introductory Definitions
Heat
Form of energy that can be transferred
from one system to another as a result of a
temperature difference.
Heat Transfer
Science that deals with the determination
of rates of energy transfer.
10
Why Study Heat Transfer?
Thermodynamics is concerned with the
amount of total heat transfer as a
system undergoes a process from one
equilibrium state to another.
However, the study of thermodynamics
gives no indication of how long it takes.
11
Why Study Heat Transfer?
Thermodynamics
Deals with equilibrium states and changes
from one system to another
Heat Transfer
Deals with systems that lack thermal
equilibrium (e.g. non-equilibrium
phenomenon).
12
Foundational Laws
However, the laws of thermodynamics
lay out the framework for studying heat
transfer.
1
st
Law Energy Equation
Rate of energy transfer into a system
equal the rate of increase of energy in the
system
2
nd
Law
Heat is transferred in the direction of
decreasing temperature.
13
Heat Transfer Direction
HOT COLD
14
1.0 Modes of Heat Transfer
15
Conduction
Transfer of energy from the more
energetic particles of a substance
to an adjacent substance with
less energetic particles, ones as a
result of interactions between the
particles
16
Conduction
Conduction can take place in
solids, liquids, or gases
In gases and liquids
conduction is due to the
collisions and diffusion of the
molecules during their
random motion.
In solids conduction is due to
the combination of vibrations
of the molecules in a lattice
and the energy transport by
free electrons
17
Conduction Equation
(Fouriers Law of Heat Conduction)
T
X
Temperature
profile
( )
( ) ( )
Thickness
Dif f erence e Temperatur Area k
x
T T
A k Q
cond
=
A
=
-
2 1
Y
X
T
1
T
2
AT
Ax
x
1
x
2
Q
x
Area (A
x
)
18
( )( )
Area Temperature difference
Rate of heat conduction
Thickness
(W)
cond
dT
Q kA
dx
=
which is called Fouriers law of heat conduction.
where the constant of proportionality k is the
thermal conductivity of the material.
19
Conduction Equation
(Fouriers Law of Heat Conduction)
Fouriers Law of Heat Conduction (1822) is:
( ) W direction x in conduction of Rate Q
x
-
~
( ) Watts
dx
dT
kA Q
x
~ =
-
( )
2
sec ~
m flow heat the
of direction the to normal area tional Cross A
|
.
|
\
|
m
C
flow heat of direction the in gradient e Temperatur
dx
dT
~
|
.
|
\
|
C m
W
material the of ty conductivi Thermal k ~
20
Thermal conductivity
The thermal conductivity (k) of a material is
defined as the rate of heat transfer through a
unit thickness of a material per unit area per
unit temperature difference.
High value for thermal conductivity - good
heat conductor
Low value - poor heat conductor or insulator.
Symbol: k
Units: W/(mC)
21
Thermal conductivity
The thermal conductivities of
gases such as air vary by a
factor of 10
4
from those of
pure metals such as copper.
Pure crystals and metals
have the highest thermal
conductivities, and gases
and insulating materials the
lowest.
22
Thermal conductivity
The thermal conductivities
of materials vary with
temperature.
The temperature
dependence of thermal
conductivity causes
considerable complexity in
conduction analysis.
A material is normally
assumed to be isotropic.
23
100 cm
x
50 cm
Conduction
(Example 1.1)
The inside and outside of the
surface of a window glass are at
20C and -5C respectively. If the
glass is 100 cm x 50 cm in size
and 1.5 cm thick, with a thermal
conductivity of 0.78 W/(mC).
Determine the heat loss through
the glass over a period of 2 hours.
20C -5C
1.5 cm
24
Conduction
(Example 1.1)
T
1
= 20 C
T
2
= -5 C
A = (100x50)= 5,000 cm
2
= 0.5 m
2
k = 0.78 W/(mC)
dx= 0.015 m
100 cm
x
50 cm
20C -5C
1.5 cm
( )
W
m
C C
m
x
T T
A k Q
C m
W
650
015 . 0
5 20
5 . 0 78 . 0
2
2 1
=
=
A
=
-
hr kW hours kW
hours over Loss Heat Total
= = 3 . 1 2 65 . 0
: 2
25
Convection
Is the mode of energy
transfer between a solid
surface and the adjacent
liquid or gas that is in
motion.
Convection involves the
combined effects of
conduction and fluid motion.
Convection = Conduction + Advection
(fluid motion)
26
Convection
Convection is commonly
classified into three sub-
modes:
Forced convection,
Natural (or free)
convection,
Change of phase
(liquid/vapor, solid/liquid,
etc.)
27
Convection Equation
(Newtons Law of Cooling)
Newtons Law of Cooling (1701) is:
( ) ( ) Watts T T A h Q
fluid wall conv
~ =
-
u
y
x
T
Heated Surface
( ) (W)
conv s s
Q hA T T
=
28
Convection Equation
(Newtons Law of Cooling)
( ) ( ) Watts T T A h Q
fluid wall conv
~ =
-
( ) W direction y in convection of Rate Q
conv
-
~
|
.
|
\
|
C m
W
t coefficien Convection h
2
~
( )
2
~ m area Surface A
( ) C e temperatur surface Wall T
wall
~
( ) C e temperatur Fluid T T
fluid
=
~
29
Convection Heat Transfer Coefficient
The convection heat transfer coefficient (h) is
not a property of a fluid (unlike k). It is an
experimentally determined parameter whose
value depends on surface geometry, fluid
motion, fluid properties, and bulk fluid
velocity.
Symbol: h
Units: W/(m
2
C)
30
31
Convection
(Example 1.2)
Atmospheric air at a
temperature of 10C flows
with a velocity 5 m/s
across a tube with an
outer diameter (OD) of 1
cm and a length of 5 cm.
The surface is maintained
at 110C.
Determine the rate of heat
flow from the tube surface
to atmospheric air if h is
85 W/(m
2
C).
AIR
1 cm
T
w
=110C
5 m
T
=10C
V = 5 m/s
h = 85 W/(m
2
C)
32
Convection
(Example 1.2)
Surface Area:
Heat Transfer per unit area:
Total Heat Flow:
( ) ( )
2
157 . 0 5 01 . 0 m m m
L D A
= =
=
t
t
( )
| |
2 2
500 , 8 10 110 85
m
W
C m
W
w
C C
T T h
A
Q
q
= =
= =
-
-
( ) ( ) W m A q Q
m
W
335 , 1 157 . 0 500 , 8
2
2
= = =
- -
33
Radiation
Unlike conduction or
convection, the transfer of
energy by radiation does
not require the presence
of an intervening medium.
Energy transfer by
radiation is the fastest
(speed of light) and
suffers no attenuation in a
vacuum.
34
The electromagnetic spectrum
The theoretical foundation of
radiation was established in 1864 by
James Maxwell (1831- 1879) of
Scotland, who postulated that
accelerated electric charges or
changing electric currents give rise to
electric and magnetic fields.
These rapidly moving fields are
called electromagnetic radiation
(can be explained as waves or
photon) - and represent the energy
emitted by matter as a result of
changes in the electronic
configurations of atoms or
molecules.
35
The electromagnetic spectrum
The heat radiated by a body is comprised of a range
of frequencies.
Thermal radiation is defined as the portion of the
spectrum between: 10
-7
and 10
-4
m.
Visible light is the portion of the spectrum
between: 3.9x10
-7
and 7.8x10
-7
m.
Solar radiation is the portion of the spectrum
between: 10
-5
and 3x10
-6
m.
Electromagnetic waves transport energy and travel
at the speed of light.
c
0
= 2.9979
x
10
8
m/s
36
Thermal radiation (10
-7
to 10
-4
m)
(3.9x10
-7
to 7.8x10
-7
m)
The electromagnetic spectrum
Solar radiation
(10
-5
to 3x10
-6
m)
All forms of matter above absolute zero (0 K) emit
thermal radiation.
Although the rate of energy emission is independent
of the surroundings, the heat transfer rate is:
Proportional to the 4
th
power of temperature of the
matter
Depends on the spatial relationships of the
surface and its surroundings.
Consequently, it is the least efficient means of
heat transfer
38
Radiation Equation
emissions- (Stefan-Boltzmann Equation)
Stefan-Boltzmann Equation:
( ) Watts T A Q
rad
~
4
=
-
o c
( ) K e temperatur surface absolute T ~
4 2
8
10 67 . 5
tan ~
K m
W
t cons Boltzmann Stef an
o
o
( ) 0 . 1 0 ~ s s c c emissivity
39
Radiation- Emissions
Stefan-Boltzman constant
( = 5.67x10
-8
W/(m
2
K
4
)
The maximum amount of radiation that can be
emitted from a surface at absolute temperature.
Blackbody
Idealized surface that emits radiation at this
maximum rate ().
power emissive Blackbody T E
b
~
4
=o
40
Radiation- Emissions
The idealized surface that emits radiation at this
maximum rate is called a blackbody.
The radiation emitted by all real surfaces is less than
the radiation emitted by a blackbody at the same
temperature, and is expressed as emissivity of the
surface (0 s s 1)
A measure of how closely the surface
approximates a blackbody
41
Greybody (real) radiation
Most objects are actually grey bodies not black
bodies.
The ratio of the total emissive power of a body to
that of a blackbody at the same temperature is
defined as the emissivity () of the body.
1 0 ; s s = c c
b
E
E
Blackbody Greybody
E
b
E
42
Blackbody (ideal) radiation
A blackbody is defined as a perfect
emitter and absorber of radiation.
At a specified temperature and
wavelength, no surface can emit more
energy than a blackbody.
A blackbody absorbs all incident radiation
energy uniformly in all directions,
regardless of wavelength and direction.
43
Absorptivity, reflectivity, and
transmission
Whenever radiant
energy is incident
upon any surface,
part may be:
Absorbed (o)
Reflected ()
Transmitted (t)
Incident
radiation
Reflected
radiation
Transmitted
radiation
Absorption
44
Radiation - Absorption
The fraction of the
radiation energy incident
on a surface that is
absorbed by the surface is
termed the absorptivity o.
Both c and o of a surface
depend on the temperature
and the wavelength of the
radiation.
0 1 o s s
45
Radiation Analysis
(Introduction)
Radiation exchange
with the surrounding
( )
4
2
4
1 s s s rad
T T A Q = o c
T
s2
46
Radiation Analysis
(Introduction)
Sun
T = 6,000 K
A
s
= 6.2x10
12
km
2
Earth (Malaysia)
T = 306 K
A
= 5.1x10
8
km
2
(0.008% of the sun)
Significant radiation heat transfer from the sun due to a large
temperature difference and large emitting surface area (A
s
).
Life on Earth depends on this!
47
Insignificant radiation heat transfer from light bulb, even
though there is a large temperature difference, due to the light
bulbs small emitting surface area (A
s
)
Radiation Analysis
(Introduction)
100-W Light bulb
T= 3,000 K
A
s
= 6.3x10
-5
m
2
Person
T= 300 K
A= 1.7 m
2
48
Why the Sky is Blue
Air molecules scatter blue
light much more than they
do red light.
At sunset, the light travels
through a thicker layer of
atmosphere which removes
most of the blue from the
natural light allowing red to
dominate.
49
On Mars it is the opposite
The Martian atmosphere
scatters red light much
more than blue light giving
it a red appearance.
At sunset the light travels
through a thicker layer of
atmosphere allowing blue
to dominate.
50
Radiation Equation
(Example 1.3)
A horizontal pipe, with a 50
mm outside diameter, is
maintained at a temperature
of 50C in a large room
where the air and wall
temperature are kept at
20C. The surface emissivity
of the steel pipe may be
taken as 0.8.
Calculate the heat loss by
radiation per unit length.
50 mm
T
1
=50C
= 0.8
L
T
2
=20C
51
Radiation Equation
(Example 1.3)
Heat loss by radiation per unit length:
K C T
K C T
293 273 20
323 273 50
2
1
= + =
= + =
( ) L L m L D A = = = 157 . 0 05 . 0 t t
( ) ( )
( ) ( ) ( ) ( ) ( ) | |
m
W
K m
W
K K m
T T D
L
Q
03 . 25
293 323 157 . 0 10 67 . 5 8 . 0
4 4
8
4
2
4
1
4 2
=
=
=
-
t o c
52
Heat Transfer Mechanisms
Now we have
covered all 3 of the
heat transfer
mechanisms.
Most real problems
will involved
combinations of
these
mechanisms.
53
54
55
Combined Example
(Example 1.4)
Air blows (at 20C) over carbon
steel [k=43 W/(m
2
C] hot plate
which is 0.5 m x 0.75 m and 20
mm thick maintained at 250C.
The convection heat transfer
coefficient is 25 W/(m
2
C) and
the heat loss from the plate
surface by radiation is 300 W.
(a) Calculate the heat transfer.
(b) The inside plate temperature.
T
1
k=43 W/(m
2
C)
Hot plate
T
w
= 250C
Energy Loss by Radiation
(300 W)
Air (T
=20C)
h= 25 W/(m
2
C)
56
Combined Example
(Example 1.4)
Heat Transfer from Newtons Law of Cooling:
Energy balance:
( )
( ) ( ) ( )
W
C C m
T T A h Q
C m
W
f w
25 . 156 , 2
20 250 75 . 0 50 . 0 25
2
2
=
=
=
-
kW kW kW
x
T
kA
Q Q Q
rad conv cond
456 . 2 3 . 0 156 . 2 = + =
A
A
+ =
- - -
57
Combined Example
(Example 1.4)
Solving for the inside plate temperature:
( ) ( )
( ) ( )
( ) ( )
C
m m
m W
A k
x W
T
C m
W
=
=
A
= A
05 . 3
5 . 0 75 . 0 43
02 . 0 456 , 2
456 , 2
2
( ) C C C
T T T
= =
A =
05 . 253 05 . 3 250
2 1
1.2(a) OZONE LAYER DEPLETION
The ozone layer is a concentration of ozone molecules in the
stratosphere. About 90% of the planet's ozone is in the ozone
layer
The ozone depletion process begins when CFCs and other
ozone-depleting substances (ODS) are emitted into the
atmosphere
It is caused by the release of chlorofluorocarbons (CFCs),
hydrofluorocarbons (HCFCs), and other ozone-depleting
substances (ODS), which were used widely as refrigerants,
insulating foams, and solvents.
A diminished ozone layer allows more radiation to reach the
Earth's surface. For people, over exposure to UV rays can lead
to skin cancer, cataracts, and weakened immune systems.
Increased UV can also lead to reduced crop yield and
disruptions in the marine food chain . (Ref: )
OZONE LAYER DEPLETION
OZONE LAYER DEPLETION
OZONE LAYER DEPLETION
What can be done?
?
1.2(b) Global Warming
Green House
Effect
GHGs
GREEN HOUSE EFFECT
Glass transmits over 90 percent of radiation in the
visible range but not the longer-wavelength (infrared
regions)
Radiation emitted by surfaces at room temperature falls
in the infrared region.
Consequently glass allows the solar radiation to enter
but does not allow the infrared radiation from the
interior surfaces to escape.
This causes a rise in the interior temperature as a
result of the energy buildup known as the greenhouse
effect,
GREEN HOUSE EFFECT
The greenhouse effect is also experienced on a
larger scale on earth.
The surface of the earth, which warms up during
the day as a result of the absorption of solar
energy, cools down at night by radiating its
energy into deep space as infrared radiation.
The combustion gases such as CO
2
and water
vapor in the atmosphere transmit the bulk of the
solar radiation but absorb the infrared radiation
emitted by the surface of the earth.
Thus, there is concern that the energy trapped
on earth will eventually cause global warming
and thus drastic changes in weather patterns.
GREEN HOUSE EFFECT
GHGs
The major greenhouse gases in the atmosphere are
carbon dioxide (CO
2
), methane, (CH
4
), nitrous oxide
(N
2
O), chlorofluorocarbons (CFCs) and ozone (O
3
).
Atmospheric water vapour (H
2
O) also makes a large
contribution to the natural greenhouse
Global atmospheric concentrations of CO
2
, CH
4
and
N
2
O have increased markedly as a result of human
activities since 1750 and now far exceed pre-
industrial values
The global increases in CO
2
concentration are due
primarily to fossil fuel use and land-use change,
while those of CH
4
and N
2
O are primarily due to
agricultural/industrial activities.
GHGs concentrations
Global Warming
MAJOR STEP IN CO
2
REDUCTION
Improve Energy Management : New (non
fossil) resources & Efficiency in utilization.
Land & Forest usage: Sustainable
Development Policy.
CARBON NEUTRAL TARGET
1.3 RENEWABLE ENERGY RESOURCES
RENEWABLE ENERGY RESOURCES
Renewable energy is energy which comes
from natural resources such as sunlight,
wind, rain, tides, and geothermal heat,
biomass etc. which are renewable (naturally
replenished).
In 2010, only about 18% of global final energy
consumption came from renewables (Ref: )
WIND ENERGY FOR
ELECTRICAL POWER GENERATION
Airflows can be used to run wind turbines.
Modern wind turbines range from around
600 kW to 5 MW of rated power. Turbines
with rated output of 1.53 MW have become
the most common for commercial use.
In Malaysia, wind energy is not technically
commercially viable resource due to low
average wind speed. may be used in micro
application.
DIRECT SOLAR ENERGY
Solar energy could be harnessed by: Actively -
Photovoltaic (PV) cells, or Passively (absorbed by
building materials etc)
Although solar energy is sufficient to meet the entire
energy needs of the world, currently it is not economical
to do so because of the low concentration of solar
energy on earth ( W/m
2
) and the high capital cost of
harnessing it due to low conversion efficiency.
High potential from emerging technologies
Biomass
Biomass - (plant material, non-fossil), organic
materials which can be burned to produce energy or
converted into fuels or other products.
Biomass is a renewable energy source because the
energy it contains comes from the sun. Through the
process of photosynthesis, plants capture the sun's
energy.
BIOMASS & BIOFUEL
Two approaches to biomass
as fuel :
growing plants specifically
for energy or using the
residue from plants used for
other things.
as bio-fuel for petroleum
subtitute
GEO-THERMAL
Geothermal
Geothermal energy is energy obtained
by tapping the heat of the earth itself,
either from kilometers deep into the
Earth's crust, or in some places of the
globe from some meters, in geothermal
heat pump
HYDRO
Hydro
Hydroelectric energy is a term usually
reserved for large-scale hydroelectric dams .
Micro hydro systems are hydroelectric power
installations that typically produce up to
100 kW of power .
Ocean energy describes all the technologies
to harness energy from the ocean/sea. This
includes marine current power, ocean
thermal energy conversion (OTEC), and tidal
power.
SUSTAINABLE DEVELOPMENT
Sustainable Development
Sustainable development is a pattern of
resource use that aims to meet human needs
while preserving the environment so that
these needs can be met not only in the
present, but also for future generations.
Sustainable development can be
conceptually devided into three constituent
parts: environmental sustainability,
economic sustainability and sociopolitical
sustainability
Energy Recovery
Most heat engines convert only approximately 20% to 50% of the
supplied energy into mechanical work whereas the remaining
energy is lost.
Many scope for technologies to recover wasted energy that takes
the form of heat discharge from exhaust or cooling water,
unburned fuel and thermal transfer.
There are many waste heat recovery systems which were designed
and used on large scale power generators. For example, some
industries that use process heat and consume a large amount of
electrical power exploit a cogeneration plant in their Rankine or
Brayton engine cycle.
Another method of optimising energy recovery in industrial power
generator is by topping the Brayton engine cycle on Rankine
engine cycle. In this combined cycle, the latent energy from the
gas turbine exhaust is recovered by transferring to the steam
energy in a waste heat exchanger (WHE) that has replaced the
boiler.
89
Renewable energy is the manifestation of solar energy in
different forms.Such energy sources include wind
energy, hydroelectric power, ocean thermal-energy,
ocean wave energy, and wood. For example, no
hydroelectric powerplant can generate electricity year
after year unless the water evaporates by absorbing
solar energy and comes back as a rainfall to replenish
the water source.
Although solar energy is sufficient to meet the entire
energy needsof the world, currently it is not economical
to do so because of the low concentration of solar
energy on earth and the high capital cost of harnessing
it.
Das könnte Ihnen auch gefallen
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (400)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (74)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (345)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (121)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- Thermal Analysis of An Aluminum Heat SinkDokument7 SeitenThermal Analysis of An Aluminum Heat Sinkapi-247715646Noch keine Bewertungen
- (2.3) C - Transfer of Thermal Energy - RadiationDokument2 Seiten(2.3) C - Transfer of Thermal Energy - Radiationzahra1Noch keine Bewertungen
- Problem9-113 (Thermal Decomposition of Parallel Plates)Dokument15 SeitenProblem9-113 (Thermal Decomposition of Parallel Plates)youga SriNoch keine Bewertungen
- PortfolioDokument35 SeitenPortfolioriya_buetNoch keine Bewertungen
- Assignment OneDokument3 SeitenAssignment OneAfif Samsun BaharunNoch keine Bewertungen
- Heat TransferDokument35 SeitenHeat Transferrukshan jayasingheNoch keine Bewertungen
- Final Report Experiment 2 Calorimetry Caubig Joseph Gary D PDFDokument7 SeitenFinal Report Experiment 2 Calorimetry Caubig Joseph Gary D PDFDenampo Ivan MikhaelNoch keine Bewertungen
- N OR S U: Egros Iental Tate NiversityDokument21 SeitenN OR S U: Egros Iental Tate NiversityKimberly Jeph TagleNoch keine Bewertungen
- Vol. 2: MaDokument1 SeiteVol. 2: Magad480Noch keine Bewertungen
- Calderas de Recuperación de Calor Con PostcombustiónDokument6 SeitenCalderas de Recuperación de Calor Con PostcombustiónaquilesanchezNoch keine Bewertungen
- Exothermic, Endothermic WebquestDokument2 SeitenExothermic, Endothermic Webquestshaylabrack1Noch keine Bewertungen
- Heat Transfer in LTV FF EvaporatorDokument10 SeitenHeat Transfer in LTV FF Evaporatorkishna009Noch keine Bewertungen
- Preliminary Heat Exchanger Design ExampleDokument4 SeitenPreliminary Heat Exchanger Design ExamplejokishNoch keine Bewertungen
- BSChE Map CurriculumDokument1 SeiteBSChE Map CurriculumJustin Paul TumaliuanNoch keine Bewertungen
- Problem Chapter 9Dokument48 SeitenProblem Chapter 9Syahid ZamaniNoch keine Bewertungen
- Hvac System TypesDokument18 SeitenHvac System TypesAnuj Bhatia100% (1)
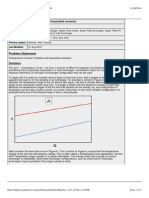
- Temperature Cross Possible and Impossible ScenarioDokument5 SeitenTemperature Cross Possible and Impossible ScenarioAhmad FarooqNoch keine Bewertungen
- Review and Advances in Heat Pipe Faghri2012Dokument18 SeitenReview and Advances in Heat Pipe Faghri2012Akash sainiNoch keine Bewertungen
- Determination of The Latent Heat of Fusion of WaterDokument3 SeitenDetermination of The Latent Heat of Fusion of WaterGlynss Samantha BanzueloNoch keine Bewertungen
- CHM1311 Lab#3Dokument13 SeitenCHM1311 Lab#3zaf77Noch keine Bewertungen
- Current Carrying Caopacity of Electrical ConductorsDokument1 SeiteCurrent Carrying Caopacity of Electrical ConductorsPhani KumarNoch keine Bewertungen
- Schauberger Inventions EnglDokument18 SeitenSchauberger Inventions Englbtkmouad100% (1)
- Lecture 01 Fundamentals of Thermodynamics 1aDokument28 SeitenLecture 01 Fundamentals of Thermodynamics 1aIsmael Torres-PizarroNoch keine Bewertungen
- UNILAB Shell & Tube Heat Exchanger Design SoftwareDokument2 SeitenUNILAB Shell & Tube Heat Exchanger Design SoftwareUnilab100% (2)
- Detailed Lesson PlanDokument6 SeitenDetailed Lesson PlanJoy Gloria91% (55)
- 1571292761168-Objective Type Question Bank For JE LGDDokument137 Seiten1571292761168-Objective Type Question Bank For JE LGDAiron Khynel U. AguilingNoch keine Bewertungen
- 6-Chapter7 - External Forced ConvectionDokument24 Seiten6-Chapter7 - External Forced ConvectionAzarul AzuwadNoch keine Bewertungen
- How Important Is Surface Area .?Dokument7 SeitenHow Important Is Surface Area .?john_serafica7104Noch keine Bewertungen
- Transport Phenomena in Food EngineeringDokument16 SeitenTransport Phenomena in Food EngineeringVane Ánady MosquedaNoch keine Bewertungen
- Refrigeration Systems Are Common in The Natural Gas Processing Industry and Processes Related To The Petroleum RefiningDokument15 SeitenRefrigeration Systems Are Common in The Natural Gas Processing Industry and Processes Related To The Petroleum RefiningMahmoud ElarabyNoch keine Bewertungen