Beruflich Dokumente
Kultur Dokumente
Black Body
Hochgeladen von
Sukhwinder Singh GillOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Black Body
Hochgeladen von
Sukhwinder Singh GillCopyright:
Verfügbare Formate
ORIGIN OF QUANTUM THEORY
BLACK BODY RADIATION
1. INTRODUCTION
2. BLACK BODY RADIATION AND ITS SPECTRUM
3. STEFANS LAW AND WIENS LAW OF RADIATION
4. RAYLEIGH-JEANS LAW
5. FAILURE OF CLASSICAL THEORY TO EXPLAIN BLACK
BODY RADIATION
6. CONCLUSION
Ref: Perspective of Modern Physics By A Beiser
Modern Physics By B L Theraja
Physics of atom By J B Rajam
BLACK BODY RADIATION
Thermal Radiation: This refers to em radiation
mainly in the IR region, by means of which heat
energy is exchanged between bodies.
Radiant Energy: All bodies at all times are
continuously emitting energy by virtue of their
temperature. This energy is called the radiant energy
or thermal radiation. It travels like visible light in the
form of electro-magnetic waves with the velocity of
light. These waves can be transmitted through
vacuum or any medium like air.
Emissive power: The emissive power of a body at a
particular temperature and for a given wavelength,
is defined as the radiant energy emitted per unit
time, per unit surface area of the body within a unit
wavelength range. If E
d is the power radiated per
unit area then E
is the emissive power.
Absorptive power: The absorptive power of a body
at a given temperature and for a given wavelength,
is defined as the ratio of radiant energy absorbed
per s , by unit surface area of the body to the total
energy falling per unit time on the same area.
BLACK BODY
A body whose absorptivity is unity for all
wavelengths is a perfect black body or simply a black
body.
Or
A body which absorbs all the incident radiation
completely irrespective of wavelength falling on it,
reflecting none and transmitting none, is called a
black body.
A black body is only an ideal concept. Lamp
black or platinum black is the nearest to such a body.
Ferys Black body :The Hollow Chamber
or Hohlraum
The hollow chamber is a good approximation
of a black body. It has a tiny aperture through
which radiation is emitted, and is immersed in a
heat bath to keep it at constant temperature.
The radiation emitted can
be detected and analyzed
with a spectrometer in
order to obtain the
spectral distribution of
the emitted energy.
1. It is found that as the temperature of a body is
raised , the body emits radiation of all wavelengths.
The color emitted by it becomes richer in waves of
shorter length.
2. The wavelength at different portions of the
spectrum can be calculated by the formula for
dispersion of prism.
3. By measuring the intensity at various
wavelengths in the whole spectrum of black body
radiation , a graph can drawn between intensity and
wavelength.
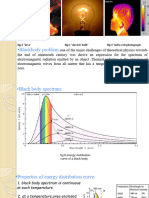
4. The fig shows plots of black body spectrum at
different temperatures.
The spectrum of the
black body radiation is
plotted for three
different temperatures,
2000 K, 1750 K, and
1250 K.
It is observed that:
1. as the temperature of the body rises, the intensity
of radiation for each wavelength increases.
2. for any one temperature, energy is distributed
continuously among the various wavelengths and is
maximum for a particular wavelength.
3. the point of maximum intensity shifts towards the
shorter wavelengths as the temperature increased.
4. the total energy of radiation for given temperature
is given by the area under the curve. The area
increases according to the forth power of absolute
temperature.
LAWS OF BLACK BODY RADIATION
1. Kirchhoffs law: It states that, at a given
temperature, the ratio of the emissive power to the
absorptive power for a given wavelength is the same
for all bodies and is equal to the emissive power of a
perfectly black body.
E
=e
/a
= constant
STEFAN-BOLTZMANN LAW
According to Stefans law, the total amount of
heat radiated by a perfectly black body per second per
unit area is directly proportional to the fourth power
of its absolute temperature
ie E T
4
or E = o T
4
where o is a constant, called Stefans constant and its
value is 5.67 x 10
8
W/m
2
K
4
.
If a black body A at
absolute temperature
T is surrounded by
another black body B
at absolute
temperature T
o
, then
amount of heat lost by black body A = o T
4
amount of heat absorbed by black body B from
black body A = o T
o
4
hence net amount of heat lost by A per second
per sq centimeter = o (T
4
- T
o
4
). This is also known as
Stefans Boltzmann law
WEINS DISPLACEMENT LAW
Wein found that as the temperature of a
black body is elevated, the spectrum retains its
general shape , but the maximum shifts towards
shorter wavelength side so that the wavelength of the
most intense radiation is inversely proportional to
the absolute temperature.
ie
m
T = constant = 0.2898 cm K.
This is known as Weins displacement law.
If the wavelength of maximum emission of the spectral
distribution of the black body is plotted over 1/T, one
obtains a straight line.
Wien's second law does a pretty good job of simulating the
behavior of the black body spectrum at short wavelengths.
It fails at longer wavelengths.
Wein obtained an expression for the
monochromatic energy density +
within an
isothermal black body enclosure in the wavelength
and +d as
+
d = C
1
-5
/ e
C2/T
d [Note: +
(c/4)=E
]
where is the wavelength , T is absolute temperature.
This formula is essentially empirical and contains two
adjustable constants C
1
and C
2
. By adjusting these two
constants Wein could explain the nature of black body
curves except at longer wavelengths.
RAYLEIGH-JEANS LAW
Rayleigh derived the radiation law based on
the following assumptions:
1. The radiant energy is due to atomic oscillators
capable of assuming all values of energy and having
an average thermal energy = kT
2. Standing e m waves exists between any two points
of the enclosure.
3. In thermal equilibrium , the average energy of the
wave equals that of the oscillators, i.e., kT.
Just like a string can vibrate to produce a
fundamental and a whole series of overtones, there
should be many modes of vibration present in the
standing waves of radiation in the cavity space. The
number of modes of vibration dn
per unit volume of
space in the wavelength range to +d is
dn
= 8t d/
4
if we multiply the above equation by the
average energy per mode (kT) then it gives the
Rayleigh-Jeans law
ie
d = = 8t kT d/
4
This law fits well for longer wavelengths but at
shorter wavelengths it tends towards infinity. This is
referred to as uv catastrophe which is predicted
from classical physics, but obviously not observed.
If the failure of Weins law
was too bad, that of R-J
law presented a crisis.
Thus classical theory was
unable to explain the black
body radiation
phenomenon.
The Planck Hypothesis
In order to explain the frequency
distribution of radiation from a hot cavity
(blackbody radiation) Planck proposed that the
atomic oscillators or resonators emit or absorb
energy in discrete units; each unit is referred to
as a quantum. The energy of a quantum is
proportional to the frequency.
To avoid the crisis presented by Rayleigh-
Jeans Law (the ultraviolet catastrophe), Planck
argued that the higher modes would be less
populated.
The quantum idea was soon seized to
explain the photoelectric effect, became part of
the Bohr theory of discrete atomic spectra, and
quickly became part of the foundation of modern
quantum theory.
PLANCKS LAW OF RADIATION
Planck was led to consider the possibility of violation
of the law of equipartition of energy on which the
classical theory is based.
Classical laws give satisfactory results at low
frequencies
The average total energy approaches kT as v
tends to zero
The discrepancy at high frequencies could be
eliminated if there exists, for some reason ,a cutoff
such that E tends to zero as v tends to infinity.
kT E
Planck realised that the average energy of standing
waves is a function of frequency E(v) having above
two properties. This is in contrast to law of
equipartition of energy
Equipartition law arises from the classical theory. The
average energy
where P (E) is the probility of finding a given particle
of a system in the range E and E+dE and is called the
Boltzmann distribution function
kT = E
kT
e
P
d P
d P
kT /
) ( ;
) (
) (
E
= E
E E
E E E
= E
}
}
Plancks Argument:
Plancks great contribution came when he realised
that he could obtain the required cutoff (E=0) by
modifying the calculation of E from P(E) by treating
the energy E as if it were a discreate variable
instead of a the continuous variable.
This can be done by writing the energy equation in
terms of sum instead of an integral.
Further, Planck assumed that the average energy at a
given frequency could take on only certain discreate
values that are integral multiples of the basic quantum.
i.e. as the set of
allowed values of energy.
Thus Planck discovered that he could obtain
when AE is small----- low frequencies and average
energy E = 0 when AE is large ----- high frequencies.
Therefore he needed to make AE an increasing
function of v.
.......... ,......... 3 , 2 , , 0 AE AE AE = E
kT = E
Numerical work showed him that he could take the
simplest possible relation between AE and v having
above properties. He assumed, AEv or A E=hv
where h is the Plancks constant=6.63 x 10
34
Js
According to Planck the average energy E is
which satisfies Plancks argument.
The energy density is given then by
This equation does agree with the experimental
results.
1
/
= E
kT h
e
h
v
v
d
e
h
d
kT h
1
8
/ 4
= +
Thus Planks quantum concept is: Radiation is not
emitted or absorbed in continuous amounts but in
discrete bundles of energy equal to hv
These bundles or packets of radiant energy are called
quanta or photons.
PROPERTIES OF PHOTON
1. Energy of a photon is represented by E=nhv where
n= 1,2,.
According to quantum mechanics E=(n+1/2) hv
The limiting value of photon energy is 1/2hv but not
zero
2. Energy of the photon is independent of intensity
3. Mass and momentum: Photon has zero rest mass
E = hv = mc
2
, therefore m=hv/c
2
And momentum p = mc= (hv/c
2
)
.
c=hv/c= h/
4. Photons are electrically neutral and hence are
unaffected by electric and magnetic fields.
Derivation of Plancks law of radiation
kT
e
P
d P
d P
kT /
) (
) (
) (
E
= E
E E
E E E
= E
}
}
In classical mechanics
the average energy of
oscillator is given by
where
is Boltzmann distribution function
Planck modified this approach by writing above in
terms of sum as
= E
E
E E
= E
kT nh
kT nh
e
e nh
P
P
/
/
) (
) (
) (
v
v
v
Put E = nhv
and P(E) = e
-nhv/kT
Expanding this series we
obtain the following
expression for the average
energy
1
/
= E
kT h
e
h
v
v
And therefore the energy density is the number of
modes of vibration X the average energy per mode
i.e.
d
e
h
d
kT h
1
8
/ 4
= +
This is Plancks
law of radiation
which explains
the correct
behaviour of
black body
radiation
phenomenon.
Das könnte Ihnen auch gefallen
- Black Body RadiationDokument33 SeitenBlack Body RadiationsindhsanamNoch keine Bewertungen
- Yearning of The SoulDokument26 SeitenYearning of The SoulAnonymous AHIfjTqLFlNoch keine Bewertungen
- Every Object of Creation Is Made of Atoms Which in Turn Connect With Each Other To Form MoleculesDokument2 SeitenEvery Object of Creation Is Made of Atoms Which in Turn Connect With Each Other To Form MoleculesShena Jalalon PenialaNoch keine Bewertungen
- GravitationDokument25 SeitenGravitationPreeti Jain100% (2)
- Module Physics (Questions)Dokument53 SeitenModule Physics (Questions)NALLATHAMBYNoch keine Bewertungen
- Phys 2o6 Sol Assign5Dokument13 SeitenPhys 2o6 Sol Assign5a1malik420Noch keine Bewertungen
- L24 Energy Work PowerDokument12 SeitenL24 Energy Work Poweri5piritiNoch keine Bewertungen
- Circuit Symbols and Circuit Diagrams: Electric Circuits: Lesson 4Dokument19 SeitenCircuit Symbols and Circuit Diagrams: Electric Circuits: Lesson 4Bry RamosNoch keine Bewertungen
- N200 EEG Lab ReportDokument19 SeitenN200 EEG Lab ReportMichael SmithNoch keine Bewertungen
- Tor PDFDokument45 SeitenTor PDFKartik PatilNoch keine Bewertungen
- Study Material/chap.2 Structure and Bonding: Kiita9Dokument36 SeitenStudy Material/chap.2 Structure and Bonding: Kiita9Saurodeep BiswasNoch keine Bewertungen
- Modern Physics (VTU) 2015-16 PDFDokument12 SeitenModern Physics (VTU) 2015-16 PDFU and me SNoch keine Bewertungen
- FALLSEM2022-23 PHY1701 ETH VL2022230106333 Reference Material I 23-09-2022 1black Body RadiationDokument11 SeitenFALLSEM2022-23 PHY1701 ETH VL2022230106333 Reference Material I 23-09-2022 1black Body RadiationTanishq AroraNoch keine Bewertungen
- Unit Iv: Quantum PhysicsDokument27 SeitenUnit Iv: Quantum PhysicsEhsaas: Mere aur TumhareNoch keine Bewertungen
- Blackbody Radiation and Atomic Emission - Unit - 07Dokument70 SeitenBlackbody Radiation and Atomic Emission - Unit - 07TEBATSONoch keine Bewertungen
- Unit - IDokument40 SeitenUnit - Icinema masterNoch keine Bewertungen
- Module 2 Complete NotesDokument18 SeitenModule 2 Complete Noteskumarravi955rNoch keine Bewertungen
- Black Body RadiationDokument12 SeitenBlack Body RadiationMahesh Lohith K.S100% (4)
- Quantum Physics: Black Body RadiationDokument7 SeitenQuantum Physics: Black Body Radiationsmcoolguy68Noch keine Bewertungen
- Black Body RadiationDokument10 SeitenBlack Body Radiationafzal786435Noch keine Bewertungen
- Theoretical BackgroundDokument2 SeitenTheoretical BackgroundPatrice Pauline TamoriaNoch keine Bewertungen
- Bose-Einstein Condensation: A PreludeDokument8 SeitenBose-Einstein Condensation: A PreludeSk. Golam Ali0% (1)
- Radiation and Its PropertiesDokument2 SeitenRadiation and Its PropertiesPraveen GuptaNoch keine Bewertungen
- CHE 301 HW 5 Abdulaziz AlhoutiDokument7 SeitenCHE 301 HW 5 Abdulaziz AlhoutiTimelessNoch keine Bewertungen
- Blackbodyradiation 170319074936Dokument38 SeitenBlackbodyradiation 170319074936LokeshNoch keine Bewertungen
- QC Lect01 Initial SubjectsDokument20 SeitenQC Lect01 Initial SubjectsCosmosianNoch keine Bewertungen
- Module 3 PPT - 221124 - 180405Dokument58 SeitenModule 3 PPT - 221124 - 180405Nikhil Rout 22BEC1020Noch keine Bewertungen
- Thermal RadiationDokument11 SeitenThermal Radiationgozombie43Noch keine Bewertungen
- Engineering Physics - : BPHY101LDokument7 SeitenEngineering Physics - : BPHY101LVenkat BalajiNoch keine Bewertungen
- Blackbody RadiationDokument9 SeitenBlackbody RadiationTitin Evania ManaluNoch keine Bewertungen
- Unit 1 Quantum Mechanics PDFDokument32 SeitenUnit 1 Quantum Mechanics PDFDeveshNoch keine Bewertungen
- Assignment On: Modern Physics Assigned By: Dr. M Ahsan Mazhar Assigned Topic: Blackbody Radiation Submitted By: M. Salman, Roll No. 49Dokument11 SeitenAssignment On: Modern Physics Assigned By: Dr. M Ahsan Mazhar Assigned Topic: Blackbody Radiation Submitted By: M. Salman, Roll No. 49Salman TabraizNoch keine Bewertungen
- Quantum NotesDokument29 SeitenQuantum NotesJayant JoshiNoch keine Bewertungen
- Black Body Radiation A: Course Number:Quantum Mechanics-1 Course Title:Phy-3201Dokument26 SeitenBlack Body Radiation A: Course Number:Quantum Mechanics-1 Course Title:Phy-3201Sheikh Emon HossainNoch keine Bewertungen
- UNIT-01: Modern PhysicsDokument28 SeitenUNIT-01: Modern PhysicsSree LakshmiNoch keine Bewertungen
- Black Body RadiationDokument9 SeitenBlack Body RadiationAnuradha Ramasundar100% (1)
- High Level Summary On Blackbody RadiationDokument7 SeitenHigh Level Summary On Blackbody RadiationMarcela ReynaNoch keine Bewertungen
- Modern Physics and Quantum Mechanics Mod-2 PDFDokument28 SeitenModern Physics and Quantum Mechanics Mod-2 PDFShreyas SeshadriNoch keine Bewertungen
- Black Body: Navigation SearchDokument32 SeitenBlack Body: Navigation SearchAishwarya RaviNoch keine Bewertungen
- Blackbody Radiation: A Presentation OnDokument9 SeitenBlackbody Radiation: A Presentation OnAakash RustagiNoch keine Bewertungen
- Black BodyDokument9 SeitenBlack BodyAakash RustagiNoch keine Bewertungen
- Unit-1 Quantum Mechanics NotesDokument13 SeitenUnit-1 Quantum Mechanics NotesPhantom's NetworkNoch keine Bewertungen
- UofT Black BodyDokument7 SeitenUofT Black BodyShayaan ZakaNoch keine Bewertungen
- Quantum1 For BEDokument19 SeitenQuantum1 For BETejaNoch keine Bewertungen
- Engineering Physics Study Material: Module - 2 Modern Physics & Quantum MechanicsDokument20 SeitenEngineering Physics Study Material: Module - 2 Modern Physics & Quantum MechanicsMonster ManNoch keine Bewertungen
- Quantum Mechanics Notes-Part 1Dokument15 SeitenQuantum Mechanics Notes-Part 1aman bhatiaNoch keine Bewertungen
- Black Body RadiationDokument14 SeitenBlack Body RadiationSaif KhanNoch keine Bewertungen
- Physics 1233Dokument11 SeitenPhysics 1233Aayush Singh100% (3)
- Fallsem2016-17 7570 RM001 14-Jul-2016 Phy1001 EthDokument12 SeitenFallsem2016-17 7570 RM001 14-Jul-2016 Phy1001 EthAnirudhNoch keine Bewertungen
- Blackbody Radiation OedDokument15 SeitenBlackbody Radiation OedrahumanNoch keine Bewertungen
- Quantum Physics: 1 Some DefinitionsDokument20 SeitenQuantum Physics: 1 Some DefinitionsDr. Shobhit SachanNoch keine Bewertungen
- Module 5: Modern Physics Lecture 23: Particle and Waves: ObjectivesDokument12 SeitenModule 5: Modern Physics Lecture 23: Particle and Waves: ObjectivesDaniyal HashmiNoch keine Bewertungen
- Module 3 - Elements of Quantum Mechanics NotesDokument40 SeitenModule 3 - Elements of Quantum Mechanics NotesMahek KhushalaniNoch keine Bewertungen
- Black Body RadiationDokument33 SeitenBlack Body RadiationSafnas Kariapper100% (1)
- Thermal Radiation: - Classical Theory - Quantum TheoryDokument9 SeitenThermal Radiation: - Classical Theory - Quantum TheoryGanantha MarsyafaNoch keine Bewertungen
- Unit3 PhyDokument66 SeitenUnit3 PhyAk JaNoch keine Bewertungen
- Thermal Radiation: - Classical Theory - Quantum TheoryDokument9 SeitenThermal Radiation: - Classical Theory - Quantum TheoryGanantha MarsyafaNoch keine Bewertungen
- Quantum Physics Prasanna BPDokument39 SeitenQuantum Physics Prasanna BPMukund KnNoch keine Bewertungen
- QM BasicsDokument12 SeitenQM Basicsh.anurag248Noch keine Bewertungen
- Planck'smanual v1Dokument12 SeitenPlanck'smanual v1spyzer.x.001Noch keine Bewertungen
- MicroElectroMechanical SystemsDokument9 SeitenMicroElectroMechanical SystemsSukhwinder Singh GillNoch keine Bewertungen
- Arch Bridge 13.08.17Dokument7 SeitenArch Bridge 13.08.17ankkeshmundra1Noch keine Bewertungen
- AGGREGDokument19 SeitenAGGREGSukhwinder Singh GillNoch keine Bewertungen
- Field Testing of CementDokument6 SeitenField Testing of CementSukhwinder Singh GillNoch keine Bewertungen
- Mem PreDokument33 SeitenMem PreSukhwinder Singh GillNoch keine Bewertungen
- Manufacture of Cement Chemical Composition Heat of Hydration Structure of Hydrated Cement Water Requirement Types of CementDokument16 SeitenManufacture of Cement Chemical Composition Heat of Hydration Structure of Hydrated Cement Water Requirement Types of CementSukhwinder Singh GillNoch keine Bewertungen
- Tutorials On Black BodyDokument2 SeitenTutorials On Black BodySukhwinder Singh GillNoch keine Bewertungen
- 6Li (d,α) α Reaction rates: Simplified representationDokument13 Seiten6Li (d,α) α Reaction rates: Simplified representationSukhwinder Singh GillNoch keine Bewertungen
- Shape Memory AlloysDokument28 SeitenShape Memory AlloysSukhwinder Singh GillNoch keine Bewertungen
- Nuc EnergyDokument40 SeitenNuc EnergySukhwinder Singh GillNoch keine Bewertungen
- Nuclear EnergyDokument10 SeitenNuclear EnergySukhwinder Singh GillNoch keine Bewertungen
- List of Text Books and Referens Books: 1. Concept of MODERN PHYSICS by Arthur Beiser Fifth EditionDokument1 SeiteList of Text Books and Referens Books: 1. Concept of MODERN PHYSICS by Arthur Beiser Fifth EditionSukhwinder Singh Gill0% (1)
- I. What Are Shape Memory Alloys?Dokument10 SeitenI. What Are Shape Memory Alloys?Sukhwinder Singh GillNoch keine Bewertungen
- Tutorial On RadioactivityDokument6 SeitenTutorial On RadioactivitySukhwinder Singh GillNoch keine Bewertungen
- Tutorials On Bohrs TheoryDokument2 SeitenTutorials On Bohrs TheorySukhwinder Singh GillNoch keine Bewertungen
- Tutorials On Bohrs TheoryDokument2 SeitenTutorials On Bohrs TheorySukhwinder Singh GillNoch keine Bewertungen
- Tutorials On Neutron CrossectionDokument1 SeiteTutorials On Neutron CrossectionSukhwinder Singh GillNoch keine Bewertungen
- Tutorials On SemiconductorsDokument4 SeitenTutorials On SemiconductorsSukhwinder Singh GillNoch keine Bewertungen
- Tutorials On Photoelectric EffectDokument1 SeiteTutorials On Photoelectric EffectSukhwinder Singh GillNoch keine Bewertungen
- Tutorials On Special RelativityDokument2 SeitenTutorials On Special RelativitySukhwinder Singh GillNoch keine Bewertungen
- Uncertainty PrincipleDokument8 SeitenUncertainty PrincipleSukhwinder Singh GillNoch keine Bewertungen
- De-Broglie WavesDokument13 SeitenDe-Broglie WavesSukhwinder Singh GillNoch keine Bewertungen
- Origin of Quantum Theory: Planck'S Law of RadiationDokument5 SeitenOrigin of Quantum Theory: Planck'S Law of RadiationSukhwinder Singh GillNoch keine Bewertungen
- Tutorials On Photoelectric EffectDokument1 SeiteTutorials On Photoelectric EffectSukhwinder Singh GillNoch keine Bewertungen
- Tutorials On Neutron CrossectionDokument1 SeiteTutorials On Neutron CrossectionSukhwinder Singh GillNoch keine Bewertungen
- Tutorial Sheet: Origin of Quantum TheoryDokument5 SeitenTutorial Sheet: Origin of Quantum TheorySukhwinder Singh Gill100% (1)
- PHYSICS1Dokument1 SeitePHYSICS1Sukhwinder Singh GillNoch keine Bewertungen
- I / I (V Constant) : PNP Transistor Aim: To Determine The Collector and Transfer CharacteristicsDokument3 SeitenI / I (V Constant) : PNP Transistor Aim: To Determine The Collector and Transfer CharacteristicsSukhwinder Singh GillNoch keine Bewertungen
- Tutorial Sheet: Origin of Quantum TheoryDokument5 SeitenTutorial Sheet: Origin of Quantum TheorySukhwinder Singh Gill100% (1)
- List of Text Books and Referens Books: 1. Concept of MODERN PHYSICS by Arthur Beiser Fifth EditionDokument1 SeiteList of Text Books and Referens Books: 1. Concept of MODERN PHYSICS by Arthur Beiser Fifth EditionSukhwinder Singh GillNoch keine Bewertungen
- The Summary of Grammatical Patterns in TOEFL-RevisedDokument22 SeitenThe Summary of Grammatical Patterns in TOEFL-RevisedSuci NadiaNoch keine Bewertungen
- Narayana DasaDokument2 SeitenNarayana DasaBrad YantzerNoch keine Bewertungen
- Mathematical Modeling of Earth's Magnetic FieldDokument21 SeitenMathematical Modeling of Earth's Magnetic FieldcrocomodxNoch keine Bewertungen
- Outerplanetary ChironDokument9 SeitenOuterplanetary ChironNina Elezovic SimunovicNoch keine Bewertungen
- Some Astro RulesDokument1 SeiteSome Astro RulesbabbansinghNoch keine Bewertungen
- Vehicle Numbers NumerologyDokument3 SeitenVehicle Numbers Numerologyravi1214100% (1)
- Katalog MikroskopDokument14 SeitenKatalog MikroskopDewi Endar YatunNoch keine Bewertungen
- Nova Praxis GM Screen PDFDokument6 SeitenNova Praxis GM Screen PDFGorkil100% (1)
- GeodynamicsDokument910 SeitenGeodynamicsDavid Herrera Guevara100% (1)
- The Anima ProblemDokument12 SeitenThe Anima ProblemjonsiekNoch keine Bewertungen
- Practice Projectiles 2Dokument4 SeitenPractice Projectiles 2fatpelicanNoch keine Bewertungen
- 04 Follow The Drinking Gourd (PDF Library)Dokument3 Seiten04 Follow The Drinking Gourd (PDF Library)Julia Villaluenga AbenojarNoch keine Bewertungen
- LAB Orbital PeriodsDokument2 SeitenLAB Orbital PeriodsAntònia Vidal Son PacsNoch keine Bewertungen
- Revision Questions in Physics 101Dokument13 SeitenRevision Questions in Physics 101lozzzzzNoch keine Bewertungen
- Online Reproductions of Lilly's Autobiography and Source TextsDokument4 SeitenOnline Reproductions of Lilly's Autobiography and Source Textsvictor chen100% (1)
- Scarborough Shoal, Also Known As Scarborough Reef, Reef (MasinlocDokument5 SeitenScarborough Shoal, Also Known As Scarborough Reef, Reef (MasinlocGrace Angelie C. Asio-SalihNoch keine Bewertungen
- Star Wars - The Rise and Fall of VadDokument83 SeitenStar Wars - The Rise and Fall of Vadjax20Noch keine Bewertungen
- Full Test 6 PDFDokument5 SeitenFull Test 6 PDFhoang lichNoch keine Bewertungen
- Mars and SaturnDokument37 SeitenMars and SaturnAdriana RamosNoch keine Bewertungen
- Major Points About AssamDokument2 SeitenMajor Points About Assamedify educationNoch keine Bewertungen
- Integrated Mathematics 4: Updated 09/15/04Dokument16 SeitenIntegrated Mathematics 4: Updated 09/15/04Neha SinghNoch keine Bewertungen
- Chinese and Western Combinations Leo (Monkey) Month Combination With Monkey YearDokument2 SeitenChinese and Western Combinations Leo (Monkey) Month Combination With Monkey YearWildlingNoch keine Bewertungen
- Prono Peru EngDokument15 SeitenProno Peru EngSantiago GuttiNoch keine Bewertungen
- New Models To Explain The Alignments of Greek TemplesDokument20 SeitenNew Models To Explain The Alignments of Greek TemplesMark CarlottoNoch keine Bewertungen
- The 11 Most Beautiful EquationsDokument5 SeitenThe 11 Most Beautiful EquationsgeronimlNoch keine Bewertungen
- Logic of English High Frequency Word List Level 3Dokument5 SeitenLogic of English High Frequency Word List Level 3ram041Noch keine Bewertungen
- Project Report - Indian Space EraDokument47 SeitenProject Report - Indian Space EraNobita TerimakiNoch keine Bewertungen
- Astrology & Vastu ModuleDokument15 SeitenAstrology & Vastu ModuleAnkitNoch keine Bewertungen
- A. J. Lustig - Erich Wasmann, Ernst Haeckel, and The Limits of ScienceDokument112 SeitenA. J. Lustig - Erich Wasmann, Ernst Haeckel, and The Limits of ScienceKein BécilNoch keine Bewertungen
- First in The WorldDokument13 SeitenFirst in The WorldABHISHEK SinghNoch keine Bewertungen