Beruflich Dokumente
Kultur Dokumente
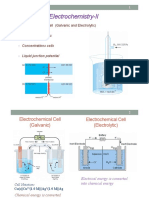
Electrodes and Potentiometry
Hochgeladen von
Megha AnandOriginalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Electrodes and Potentiometry
Hochgeladen von
Megha AnandCopyright:
Verfügbare Formate
Electrodes and Potentiometry
Introduction
1.) Potentiometry
Use of Electrodes to Measure Voltages that Provide Chemical Information
- Various electrodes have been designed to respond selectively to specific analytes
Use a Galvanic Cell
- Unknown solution becomes a -cell
- Add Electrode that transfers/accepts
electrons from unknown analyte
- Connect unknown solution by salt
bridge to second -cell at fixed
composition and potential
Indicator Electrode: electrode that
responds to analyte and donates/accepts
electrons
Reference Electrode: second cell at a
constant potential
Cell voltage is difference between the
indicator and reference electrode
Binding generates
potential difference.
Electrodes and Potentiometry
Introduction
2.) Example
A Heparin Sensor
- Voltage response is proportional to heparin concentration in blood
- Sensor is selective for heparin
Negatively charged heparin
binds selectively to positively
charged membrane.
heparin
Potential is
proportional to
[heparin]
Electrodes and Potentiometry
Reference Electrodes
1.) Overview
Potential change only dependent on one cell concentrations
Reference electrode is fixed or saturated doesnt change!
Reference electrode,
[Cl
-
] is constant
Potential of the cell
only depends on
[Fe
2+
] & [Fe
3+
]
Pt wire is indicator
electrode whose
potential responds
to [Fe
2+
]/[Fe
3+
]
{ } ] Cl log[ . .
] Fe [
] Fe [
log
.
. E
cell
+
+
|
|
.
|
\
|
= 05916 0 222 0
1
05916 0
771 0
3
2
Unknown solution of
[Fe
2+
] & [Fe
3+
]
Electrodes and Potentiometry
Reference Electrodes
2.) Silver-Silver Chloride Reference Electrode
Convenient
- Common problem is porous plug becomes clogged
E
o
= +0.222 V
Activity of Cl
-
not 1E
(sat,KCl)
= +0.197 V
Electrodes and Potentiometry
Reference Electrodes
3.) Saturated Calomel Reference Electrode (S.C.E)
Saturated KCl maintains constant [Cl
-
] even with
some evaporation
Standard hydrogen electrodes are cumbersome
- Requires H
2
gas and freshly prepared Pt surface
E
o
= +0.268 V
Activity of Cl
-
not 1E
(sat,KCl)
= +0.241 V
Electrodes and Potentiometry
Reference Electrodes
4.) Observed Voltage is Reference Electrode Dependant
The observed potential depends on the choice of reference electrode
- Silver-silver chloride and calomel have different potentials
Use Reference Scale to convert between Reference Electrodes
Observed potential relative to SCE
Observed potential relative to Ag|AgCl
Observed potential relative to SHE
Electrodes and Potentiometry
Junction Potential
1.) Occurs Whenever Dissimilar Electrolyte Solutions are in Contact
Develops at solution interface (salt bridge)
Small potential (few millivolts)
Junction potential puts a fundamental limitation on the accuracy of direct
potentiometric measurements
- Dont know contribution to the measured voltage
Again, an electric potential is generated by a separation of charge
Different ion mobility results
in separation in charge
Electrodes and Potentiometry
Indicator Electrodes
1.) Two Broad Classes of Indicator Electrodes
Metal Electrodes
- Develop an electric potential in response to a redox reaction at the metal
surface
Ion-selective Electrodes
- Selectively bind one type of ion to a membrane to generate an electric
potential
Remember an electric potential is generated by a separation of charge
Electrodes and Potentiometry
Indicator Electrodes
2.) Metal Electrodes
Platinum
- Most common metal indicator electrode
- Inert: does not participate in many chemical reactions
- Simply used to transmit electrons
Other electrodes include Gold and Carbon
Metals (Ag, Cu, Zn, Cd, Hg) can be used to monitor their aqueous ions
- Most metals are not useable
- Equilibrium not readily established at the metal surface
E
+
o
= +799 V
E
(sat,KCl)
= +0.241 V
Reaction at Ag indicator electrode:
Reaction at Calomel reference electrode:
{ } 241 0
1
1
05916 0
799 0 .
] Ag [
log
.
. E E E
cell
|
|
.
|
\
|
= =
+
+
Potential of Ag
indicator electrode
Cell voltage changes as a function of [Ag
+
]
Example:
Cell Potential from Nernst Equation:
C
+
diffuses across the membrane due to
concentration gradient resulting in charge
difference across membrane
Electrodes and Potentiometry
Indicator Electrodes
4.) Ion-Selective Electrodes
Responds Selectively to one ion
- Contains a thin membrane
capable of only binding the
desired ion
Does not involve a redox process
Membrane contains a ligand (L) that
specifically and tightly binds analyte
of interest (C
+
)
A difference in the concentration of C
+
exists across the outer membrane.
The counter-ions (R
-
,A
-
) cant cross the
membrane and/or have low solubility in
membrane or analyte solution
Remember an electric potential is generated by a separation of charge
Potential across outer membrane depends on
[C+] in analyte solution
Electrodes and Potentiometry
Indicator Electrodes
4.) Ion-Selective Electrodes
Responds Selectively to one ion
- Contains a thin membrane
capable of only binding the
desired ion
Does not involve a redox process
Remember an electric potential is generated by a separation of charge
Potential across inner membrane depends on [C+]
in filling solution, which is a known constant
C
+
diffuses across the membrane due to
concentration gradient resulting in charge
difference across membrane
A difference in the concentration of C
+
exists across the inner membrane.
inner outer
E E E =
Electrode potential is determined by the potential
difference between the inner and outer membranes:
where E
inner
is a constant and E
outer
depends on the
concentration of C
+
in analyte solution
] [ constant
+
+ = C log
n
.
E
05916 0
Electrodes and Potentiometry
Indicator Electrodes
4.) Ion-Selective Electrodes
Responds Selectively to one ion
- Contains a thin membrane
capable of only binding the
desired ion
Does not involve a redox process
Electrode Potential is defined as:
where [C
+
] is actually the activity of the analyte and
n is the charge of the analyte
Electrodes and Potentiometry
pH Electrodes
1.) pH Measurement with a Glass Electrode
Glass electrode is most common ion-selective electrode
Combination electrode incorporates both glass and
reference electrode in one body
Ag(s)|AgCl(s)|Cl
-
(aq)||H
+
(aq,outside) H
+
(aq,inside),Cl
-
(aq)|AgCl(s)|Ag(s)
Outer reference
electrode
[H
+
] outside
(analyte solution)
[H
+
] inside
Inner reference
electrode
Glass membrane
Selectively binds H
+
Electric potential is generated by [H
+
] difference across glass membrane
Electrodes and Potentiometry
pH Electrodes
2.) Glass Membrane
Irregular structure of silicate lattice
Cations (Na
+
) bind
oxygen in SiO
4
structure
The Gel Layer
The glass has a lithium
silicate skeleton that
forms a thin hydrated
layer on both sides of the
membrane.
Ions can penetrate this
thin layer and alter the
electrochemical potential.
Without the hydrated
layer no pH
measurements would be
possible
The structure of the glass
has been optimised so
that virtually only H
+
ions
can enter the gel layer
Electrodes and Potentiometry
pH Electrodes
2.) Glass Membrane
Two surfaces of glass swell as they absorb water
- Surfaces are in contact with [H
+
]
Electrodes and Potentiometry
pH Electrodes
2.) Glass Membrane
H
+
diffuse into glass membrane and replace Na
+
in hydrated gel region
- Ion-exchange equilibrium
- Selective for H
+
because H
+
is only ion that binds significantly to the
hydrated gel layer
H (0.05916) constant p E | =
Charge is slowly carried
by migration of Na+
across glass membrane
Potential is determined
by external [H
+
]
Constant and b are measured when electrode is calibrated with solution of known pH
Electrodes and Potentiometry
pH Electrodes
3.) Calibration
A pH electrode should be calibrated with two or more standard buffers
before use.
pH of the unknown should lie within the range of the standard buffers
Measured voltage is correlated
with a pH, which is then used to
measure an unknown.
Electrodes and Potentiometry
pH Electrodes
4.) Errors in pH Measurements
Standards
- pH measurements cannot be more accurate than standards (0.01)
Junction potential
- If ionic strengths differ between analyte and standard buffer, junction potential
will differ resulting in an error of 0.01
Junction Potential Drift
- Caused by slow changes in [KCl] and [AgCl] re-calibrate!
Sodium Error
- At very low [H
+
], electrode responds to Na
+
and the apparent pH is lower than
the true pH
Acid Error
- At high [H
+
], the measured pH is higher than actual pH, glass is saturated
Equilibration Time
- Takes ~30s to minutes for electrode to equilibrate with solution
Hydration of glass
- A dry electrode will not respond to H
+
correctly
Temperature
- Calibration needs to be done at same temperature of measurement
Cleaning
- Contaminates on probe will cause reading to drift until properly cleaned or
equilibrated with analyte solution
Electrodes and Potentiometry
pH Electrodes
4.) Errors in pH Measurements
pH measurements are accurate to 0.02 pH units
Larger errors occur at high
and low pH readings
Electrodes and Potentiometry
Other Ion-Selective Electrodes
1.) Solid-State Electrode
Based on an inorganic crystal
- Fluoride electrode: LaF
3
crystal doped with Eu
2+
F
-
migrates across crystal by jumping
into crystal vacancies caused by Eu
2+
Potential caused by charge imbalance from migrating ion across membrane
= F (0.05916) constant p E |
Electrodes and Potentiometry
Other Ion-Selective Electrodes
2.) Liquid-Based Ion-Selective Electrodes
Similar to solid-state electrode
- Hydrophobic membrane impregnated with hydrophobic ion exchanger
- Hydrophobic ion exchanger selective for analyte ion
+
+ =
2
pCa E )
2
0.05916
( constant |
Binds Ca
+2
Hydrophobic solvent
Hydrophobic counter-ion
Electrodes and Potentiometry
Other Ion-Selective Electrodes
2.) Liquid-Based Ion-Selective
Electrodes
Remember: ion-selective electrodes
create a potential from a charge
imbalance caused by analyte ion
migration across membrane
Electrodes and Potentiometry
Other Ion-Selective Electrodes
3.) Compound Electrodes
Conventional electrode surrounded by a membrane that isolates or generates
the analyte to which the electrode responds
pH electrode surrounded by membrane
permeable to CO
2
.
As CO
2
passes through membrane and
dissolves in solution, pH changes.
pH change is an indirect measure of CO
2
concentration
Electrodes and Potentiometry
Other Ion-Selective Electrodes
4.) Standard Addition
Corrects for analyte dissolved in complex or unknown matrix
- Blood, urine, biomass, etc
Procedure:
1. Measure potential for unknown analyte solution
2. Add small (known) volume of a standard solution
3. Measure new potential
4. Repeat and graph data
s s
S / k
x o
S / k S / E
s o
V c c V ) V V ( 10 10 10 + = +
y
b
m
x
where:
V
o
is the initial volume
V
s
is the added volume
E is the measured potential
c
x
is the unknown concentration
c
s
is the standard concentration
s is a constant (|RT/nF)ln10
Other Ion-Selective Electrodes
4.) Standard Addition
Corrects for analyte dissolved in complex or unknown matrix
- Blood, urine, biomass, etc
Procedure:
5. x-intercept yields the unknown (c
x
) concentration
Electrodes and Potentiometry
s
x o
c
c V
m
b
x = = intercept
Only unknown
Das könnte Ihnen auch gefallen
- PotentiometryDokument27 SeitenPotentiometryShafique Ahmed100% (2)
- Lecture 7Dokument53 SeitenLecture 7Chau MaiNoch keine Bewertungen
- ISO-9963-1-1994 Alkalinity in WaterDokument9 SeitenISO-9963-1-1994 Alkalinity in WaterJOSEPH OMONDINoch keine Bewertungen
- Astm E70Dokument6 SeitenAstm E70DIEGONoch keine Bewertungen
- Chapter 15 of GlobeDokument26 SeitenChapter 15 of GlobeSyed Zakir Hussain ZaidiNoch keine Bewertungen
- POTENTIOMETRYDokument6 SeitenPOTENTIOMETRYDavid HendersonNoch keine Bewertungen
- Lecture 3 - Potentiometry 1Dokument22 SeitenLecture 3 - Potentiometry 1Abd El-Fattah Mohamed OufNoch keine Bewertungen
- Chapter 22: Introduction To Electroanalytical ChemistryDokument29 SeitenChapter 22: Introduction To Electroanalytical ChemistryMohammad Kabir HossainNoch keine Bewertungen
- Electrochemistry Part 2 NoteDokument28 SeitenElectrochemistry Part 2 NoteMuhdLuqmanNoch keine Bewertungen
- Electroanalytical Methods and Potentiometry PDFDokument15 SeitenElectroanalytical Methods and Potentiometry PDFLilato ChanganiNoch keine Bewertungen
- Lec 8bDokument16 SeitenLec 8bdavidolalere7Noch keine Bewertungen
- Potentiometry 2021Dokument47 SeitenPotentiometry 2021Adina IqbalNoch keine Bewertungen
- Potentiometry: E E + (0.0592/n) Log CDokument7 SeitenPotentiometry: E E + (0.0592/n) Log CBen AbellaNoch keine Bewertungen
- Potentiometry: Kimia Analitik Universitas PertaminaDokument40 SeitenPotentiometry: Kimia Analitik Universitas PertaminaVincentius EkyNoch keine Bewertungen
- Types of ElecrodesDokument27 SeitenTypes of ElecrodesakshayNoch keine Bewertungen
- PotentiometryDokument46 SeitenPotentiometryMohammad Sabir HussainNoch keine Bewertungen
- PotentiometryDokument10 SeitenPotentiometryAnisah RachmawatiNoch keine Bewertungen
- Potentiometry FinalDokument27 SeitenPotentiometry FinalDeepak shahNoch keine Bewertungen
- Types of ElectrodesDokument24 SeitenTypes of ElectrodesPUBG ASSASIN தமிழ் ASASSINNoch keine Bewertungen
- Electrochemical-Cells Kec PDFDokument10 SeitenElectrochemical-Cells Kec PDFsachinNoch keine Bewertungen
- 1 Introduction Potentiometry With Reference and Indicator ElectrodesDokument110 Seiten1 Introduction Potentiometry With Reference and Indicator ElectrodesSzaki Flores VillaflorNoch keine Bewertungen
- Introduction and Overview of Electrode ProcessDokument33 SeitenIntroduction and Overview of Electrode ProcessGIRMA SELALE GELETANoch keine Bewertungen
- Potensiometri: Igaa SeptiariDokument27 SeitenPotensiometri: Igaa SeptiariGung AriNoch keine Bewertungen
- Susmita PDFDokument14 SeitenSusmita PDFRohan KarNoch keine Bewertungen
- U, I, R F (C) U: Voltage, I: Current, R: ResistanceDokument23 SeitenU, I, R F (C) U: Voltage, I: Current, R: ResistanceAmaya GalindoNoch keine Bewertungen
- Chapter 21-Potentiometry V2Dokument43 SeitenChapter 21-Potentiometry V2S. MartinezNoch keine Bewertungen
- Potentiometric Measurements Galvanic or Voltaic Cell: ElectrolyticDokument21 SeitenPotentiometric Measurements Galvanic or Voltaic Cell: ElectrolyticAmr GamalNoch keine Bewertungen
- Lecture 9, Potentiometgnfgry, PakinazDokument52 SeitenLecture 9, Potentiometgnfgry, PakinazMostufa KhedrawyNoch keine Bewertungen
- Mod 5 Revision Guide 3. RedoxdcDokument4 SeitenMod 5 Revision Guide 3. RedoxdcJillian PeteNoch keine Bewertungen
- 2 - Potentiometry 2013Dokument52 Seiten2 - Potentiometry 2013AtikDwiOktavianiNoch keine Bewertungen
- Referentne ElektrodeDokument26 SeitenReferentne ElektrodeZlata JašarevićNoch keine Bewertungen
- 3 PotentiometryDokument11 Seiten3 Potentiometry175-44-Faraz HussainNoch keine Bewertungen
- Biomedical Electronics & BioinstrumentationDokument44 SeitenBiomedical Electronics & BioinstrumentationashadoitNoch keine Bewertungen
- Potentiometry 1Dokument39 SeitenPotentiometry 1eswarNoch keine Bewertungen
- Chapter 4 Electrochemical TechniquesDokument49 SeitenChapter 4 Electrochemical TechniquestufabededaNoch keine Bewertungen
- PotentiometryDokument69 SeitenPotentiometrymianNoch keine Bewertungen
- Science Chemistry Teaching Resources Documents POTENTIOMETRYDokument60 SeitenScience Chemistry Teaching Resources Documents POTENTIOMETRYamol Akolkar ( amolpc86)Noch keine Bewertungen
- ElectroDokument13 SeitenElectrodulalsushant3Noch keine Bewertungen
- Fourth Hour ContinutaionDokument23 SeitenFourth Hour ContinutaionMegan RiveraNoch keine Bewertungen
- Electochemistry PDFDokument29 SeitenElectochemistry PDFAnshu KarmacharyaNoch keine Bewertungen
- Introduction To ElectrochemistryDokument40 SeitenIntroduction To ElectrochemistryAngates1100% (2)
- Electroanalytical ChemistryDokument31 SeitenElectroanalytical Chemistryyouni_2005Noch keine Bewertungen
- Intro To Electroanalytical ChemistryDokument38 SeitenIntro To Electroanalytical Chemistryarun231187Noch keine Bewertungen
- Sathyabama UniversityDokument20 SeitenSathyabama UniversityMathavaraja JeyaramanNoch keine Bewertungen
- Potentiometry - AmperometryDokument73 SeitenPotentiometry - AmperometryEva Apriliyana RizkiNoch keine Bewertungen
- 2marks QuestionDokument7 Seiten2marks QuestionjranjithsinghNoch keine Bewertungen
- ElectrochemistryDokument5 SeitenElectrochemistrydanielmahsaNoch keine Bewertungen
- Potentiometry: Dr. Kathlia A. de Castro-Cruz Analytical Chemistry For CheDokument59 SeitenPotentiometry: Dr. Kathlia A. de Castro-Cruz Analytical Chemistry For CheCyrus VizonNoch keine Bewertungen
- 2 - Potentiometry 2013Dokument52 Seiten2 - Potentiometry 2013KurniaAnisaNoch keine Bewertungen
- Potentiometry & Ion Selective Electrode: DR - Ruchi Gokani Dept of Biochemistry S.B.K.S.M.I.R.CDokument36 SeitenPotentiometry & Ion Selective Electrode: DR - Ruchi Gokani Dept of Biochemistry S.B.K.S.M.I.R.Cpalla gopalNoch keine Bewertungen
- PHLEbDokument12 SeitenPHLEbMigs BernalNoch keine Bewertungen
- Chapter 23Dokument21 SeitenChapter 23Amsalu WalelignNoch keine Bewertungen
- Week 4 - Basic Concept of Corrosion - Part 1Dokument75 SeitenWeek 4 - Basic Concept of Corrosion - Part 1Araasu EgambaramNoch keine Bewertungen
- NotesDokument20 SeitenNotesP I X Ξ LNoch keine Bewertungen
- NotesDokument20 SeitenNotesP I X Ξ LNoch keine Bewertungen
- Potentiometry: By: Arif SultanDokument14 SeitenPotentiometry: By: Arif SultanMehtab Ahmed AbbasiNoch keine Bewertungen
- B. Electrochemistry 2 1Dokument37 SeitenB. Electrochemistry 2 1Dank CoderNoch keine Bewertungen
- Week 5. ElectrochemistryDokument55 SeitenWeek 5. ElectrochemistrynorsiahNoch keine Bewertungen
- Revision Note Ajc-1Dokument8 SeitenRevision Note Ajc-1Madhavilatha LoganathanNoch keine Bewertungen
- Chapter 22: Introduction To Electroanalytical ChemistryDokument29 SeitenChapter 22: Introduction To Electroanalytical ChemistryMedina KimJunelNoch keine Bewertungen
- Complete Electronics Self-Teaching Guide with ProjectsVon EverandComplete Electronics Self-Teaching Guide with ProjectsBewertung: 3 von 5 Sternen3/5 (2)
- Micro ProcessorDokument442 SeitenMicro ProcessorMegha Anand0% (1)
- SR - No Tag No. System Quantity Range Units Size/Make Type of Instrument ServiceDokument1 SeiteSR - No Tag No. System Quantity Range Units Size/Make Type of Instrument ServiceMegha AnandNoch keine Bewertungen
- P&ID Melt Clarification SystemDokument1 SeiteP&ID Melt Clarification SystemMegha AnandNoch keine Bewertungen
- Introduction To ElectrophoresisDokument52 SeitenIntroduction To ElectrophoresisMegha AnandNoch keine Bewertungen
- Environment 23Dokument10 SeitenEnvironment 23Megha AnandNoch keine Bewertungen
- WaterDokument4 SeitenWaterMegha AnandNoch keine Bewertungen
- Ion Exchange ChromatographyDokument18 SeitenIon Exchange ChromatographyPriya DharshiniNoch keine Bewertungen
- Factrs Affctng Acrcy of WavemtrDokument1 SeiteFactrs Affctng Acrcy of WavemtrMegha AnandNoch keine Bewertungen
- New Microsoft Office Word Document - BiodieselDokument1 SeiteNew Microsoft Office Word Document - BiodieselMegha AnandNoch keine Bewertungen
- Intro To BMIDokument57 SeitenIntro To BMIMegha AnandNoch keine Bewertungen
- Megha Anand - ResumeDokument2 SeitenMegha Anand - ResumeMegha AnandNoch keine Bewertungen
- PhosphateDokument13 SeitenPhosphateMohit AnandNoch keine Bewertungen
- Manual PHmetro AdwaDokument2 SeitenManual PHmetro AdwaDavid VelasquezNoch keine Bewertungen
- Multiple Choice: TrueorfalseDokument4 SeitenMultiple Choice: TrueorfalseMary Francia Rico100% (1)
- Field Acid Sulfate TestsDokument6 SeitenField Acid Sulfate TestsAnton ManojNoch keine Bewertungen
- Technical Note: PH Measurement and Temperature CompensationDokument1 SeiteTechnical Note: PH Measurement and Temperature CompensationEliana NiñoNoch keine Bewertungen
- E - Science Sba DraftDokument25 SeitenE - Science Sba DraftLashaunte Hodge HobsonNoch keine Bewertungen
- Chemical Examination of Urine: LearningobjectivesDokument38 SeitenChemical Examination of Urine: LearningobjectivesWho KnowsNoch keine Bewertungen
- Analog Output Settings For Instruments RevDokument5 SeitenAnalog Output Settings For Instruments RevJon GonzalNoch keine Bewertungen
- Determination of PH Exp No: 3 Date AimDokument2 SeitenDetermination of PH Exp No: 3 Date AimkuthappadyNoch keine Bewertungen
- Acid-Base Titrations: The Complete Applications PackageDokument55 SeitenAcid-Base Titrations: The Complete Applications PackageoldpenguinNoch keine Bewertungen
- PH Electrode Cleaning & Maintenance GuideDokument4 SeitenPH Electrode Cleaning & Maintenance Guidejonathan_calixto_4Noch keine Bewertungen
- Oakton PH 5+, PH 6+, Ion 6+ Instruction ManualDokument28 SeitenOakton PH 5+, PH 6+, Ion 6+ Instruction ManualAngel ReyesNoch keine Bewertungen
- Manual ST5000 Potenciometro EspañolDokument80 SeitenManual ST5000 Potenciometro EspañolBrandon GonzalezNoch keine Bewertungen
- Collected Coursework Problems in Biochemical Engineering BlanchDokument6 SeitenCollected Coursework Problems in Biochemical Engineering Blanchbcqy65mx100% (2)
- Basic Circuits - BMI LabDokument55 SeitenBasic Circuits - BMI LabkaranipgrNoch keine Bewertungen
- LL Solvotrode: GeneralDokument2 SeitenLL Solvotrode: GeneralcelmorcelliNoch keine Bewertungen
- University of Sulaimani College of Engineering Water Resource DepartmentDokument10 SeitenUniversity of Sulaimani College of Engineering Water Resource DepartmentSharo Shwan OthmanNoch keine Bewertungen
- SOP PHDokument2 SeitenSOP PHrancidNoch keine Bewertungen
- Cat Gen HannaDokument708 SeitenCat Gen HannaRamona BarnaNoch keine Bewertungen
- The Acidic EnvironmentDokument47 SeitenThe Acidic EnvironmentJeremy ElvinNoch keine Bewertungen
- Hach PH Meter ManualDokument14 SeitenHach PH Meter ManualSreenath RajNoch keine Bewertungen
- VTU Chemistry Lab Revision SheetDokument3 SeitenVTU Chemistry Lab Revision SheetSHREYAS A HOMBALNoch keine Bewertungen
- Guide To PH Analysis For Lab Ebook V2Dokument24 SeitenGuide To PH Analysis For Lab Ebook V2Pratik JainNoch keine Bewertungen
- ASTM-D6276-99a-Using PH To Estimate The Soil-Lime Proportion Requirement For Soil StabilizationDokument4 SeitenASTM-D6276-99a-Using PH To Estimate The Soil-Lime Proportion Requirement For Soil StabilizationMiguel RochaNoch keine Bewertungen
- Enactus Liquid Soap ProjectDokument40 SeitenEnactus Liquid Soap ProjectWendellReeceFrank100% (1)
- PH计CT-6023 Instruction ManualDokument3 SeitenPH计CT-6023 Instruction ManualAnonymous kBjvdERRQNoch keine Bewertungen
- Aqua ManualDokument127 SeitenAqua ManualNarasimha MurthyNoch keine Bewertungen
- Hanna Instruments General CatalogDokument1.012 SeitenHanna Instruments General CatalogRafael Ignacio OrdenNoch keine Bewertungen
- PH METERS, HYDROLYSIS, AND BUFFER CAPACITYDokument10 SeitenPH METERS, HYDROLYSIS, AND BUFFER CAPACITYnermeen ahmedNoch keine Bewertungen