Beruflich Dokumente
Kultur Dokumente
Chapter-12 EDTA Titrations
Hochgeladen von
jignesh_panOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Chapter-12 EDTA Titrations
Hochgeladen von
jignesh_panCopyright:
Verfügbare Formate
EDTA Titrations
Introduction
1.) Metal Chelate Complexes
Any reagent which reacts with an analyte in a known ratio and with a large
equilibrium constant can potentially be used in a titration.
Complexation Titrations are based on the reaction of a metal ion with a
chemical agent to form a metal-ligand complex.
Metal
Ligand
Metal-Ligand Complex
Metal Lewis Acid or Electron-pair acceptor
Ligand Lewis Base or Electron-pair donor
Note: multiple atoms from
EDTA are binding Mn
2+
EDTA Titrations
Introduction
1.) Metal Chelate Complexes
Complexation Titrations are essentially a Lewis acid-base reaction, in which
an electron pair is donated from one chemical to another
The ligands used in complexometric titrations are also known as chelating
agents.
- Ligand that attaches to a metal ion through more than one ligand atom
Most chelating agents contain N or O
- Elements that contain free electron pairs that may be donated to a metal
Fe-DTPA Complex
EDTA Titrations
Metal Chelation in Nature
1.) Potassium Ion Channels in Cell Membranes
Electrical signals are essential for life
Electrical signals are highly controlled by the selective passage of ions across
cellular membranes
- Ion channels control this function
- Potassium ion channels are the largest and most diverse group
- Used in brain, heart and nervous system
Current Opinion in Structural Biology 2001, 11:408414
Opening of potassium channel allows K
+
to exit cell
and change the electrical potential across membrane
K
+
channel spans membrane
channel contains
pore that only
allows K
+
to pass
K
+
is chelated by O
in channel
http://www.bimcore.emory.edu/home/molmod/Wthiel/Kchannel.html
EDTA Titrations
Metal Chelate Complexes
1.) Formation Constant (K
f
)
The equilibrium constant for the reaction between a metal ion (M
+n
) and a
chelating agent (L
-P
) is known as a formation constant or stability constant.
Applying different and specific names to the general equilibrium constant is a
common occurrence
- Solubility (K
sp
), acid-base (K
a
, K
b
), water dissociation (K
w
), etc
Chelate effect: ability of multidentate ligands to form stronger metal
complexes compared to monodentate ligands.
K
f
= 8x10
9
K
f
= 4x10
9
2 ethylenediamine molecules binds tighter than 4 methylamine molecules
EDTA Titrations
Metal Chelate Complexes
2.) Chelate Effect
Usually chelating agents with more than one electron pair to donate will form
stronger complexes with metal ions than chelating agents with only one
electron pair.
- Typically more than one O or N
- Larger K
f
values
Multidentate ligand: a chelating agent with more than one free electron pair
- Stoichiometry is 1:1 regardless of the ion charge
Monodentate ligand: a chelating agent with only one pair of free electrons
Multidentate ligand that binds radioactive metal attached
to monoclonal antibody (mAb).
mAb is a protein that binds to a specific feature on a
tumor cell delivering toxic dose of radiation.
EDTA Titrations
EDTA
1.) EDTA (Ethylenediaminetetraacetic acid)
One of the most common chelating agents used for complexometric titrations
in analytical chemistry.
EDTA has 6 nitrogens & oxygens in its structure giving it 6 free electron pairs
that it can donate to metal ions.
- High K
f
values
- 6 acid-base sites in its structure
EDTA Titrations
EDTA
2.) Acid-Base Forms
EDTA exists in up to 7 different acid-base forms depending on the solution
pH.
The most basic form (Y
4-
) is the one which primarily reacts with metal ions.
EDTA-Mn Complex
EDTA Titrations
EDTA
2.) Acid-Base Forms
Fraction (o) of the most basic form of EDTA (Y
4-
) is defined by the H
+
concentration and acid-base equilibrium constants
| | EDTA
Y
Y HY Y H Y H Y H Y H Y H
Y
4
Y
4 3 2
2 3 4 5
2
6
4
Y
4
4
] [
] [ ] [ ] [ ] [ ] [ ] [ ] [
] [
+ +
=
+ + + + + +
=
o
o
Fraction (o) of EDTA in the form Y
4-
:
where [EDTA] is the total concentration of all free EDTA species in solution
} ] [ ] [ ] [ ] [ ] [ ] {[
2 3 4 5 6
6 5 4 3 2 1 5 4 3 2 1 4 3 2 1 3 2 1 2 1 1
6 5 4 3 2 1
Y
K K K K K K K K K K K H K K K K H K K K H K K H K H H
K K K K K K
4
+ + + + + +
=
+ + + + + +
o
o
Y4-
is depended on the pH of the solution
EDTA Titrations
EDTA
3.) EDTA Complexes
The basic form of EDTA (Y
4-
) reacts with most metal ions to form a 1:1
complex.
- Other forms of EDTA will also chelate metal ions
Recall: the concentration of Y
4-
and the total concentration of EDTA is
solution [EDTA] are related as follows:
] ][ [
] [
+
=
4 n
4 n-
f
Y M
MY
K
Note: This reaction only involves Y
4-
, but not the other forms of EDTA
| | EDTA Y
4
Y
4
=
o ] [
where o
Y4-
is dependent on pH
EDTA Titrations
EDTA
3.) EDTA Complexes
The basic form of EDTA (Y
4-
) reacts with most metal ions to form a 1:1
complex.
EDTA Titrations
EDTA
3.) EDTA Complexes
Substitute [Y
4-
] into K
f
equation
If pH is fixed by a buffer, then o
Y4-
is a constant that can be combined with K
f
] ][ [
] [
+
=
4 n
4 n-
f
Y M
MY
K
| | EDTA Y
4
Y
4
=
o ] [
] [ ] [
] [
- 4
Y
EDTA M
MY
K
n
4 n-
f
o
+
=
where [EDTA] is the total
concentration of EDTA added
to the solution not bound to
metal ions
] ][ [
] [
- 4
Y
EDTA M
MY
K K K
n
4 n-
f
'
f
+
= = = o
Conditional or effective formation constant:
(at a given pH)
EDTA Titrations
EDTA
3.) EDTA Complexes
Assumes the uncomplexed EDTA were all in one form
- 4
Y
o
f
'
f
K K =
at any pH, we can find o
Y4-
and evaluate K
f
EDTA Titrations
EDTA
4.) Example:
What is the concentration of free Fe
3+
in a solution of 0.10 M Fe(EDTA)
-
at pH
8.00?
EDTA Titrations
EDTA
5.) pH Limitation
Note that the metal EDTA complex becomes less stable as pH decreases
- K
f
decreases
- [Fe
3+
] = 5.4x10
-7
at pH 2.0 -> [Fe
3+
] = 1.4x10
-12
at pH 8.0
In order to get a complete titration (K
f
10
6
), EDTA requires a certain
minimum pH for the titration of each metal ion
End Point becomes less distinct as pH is
lowered, limiting the utility of EDTA as a titrant
EDTA Titrations
EDTA
5.) pH Limitation
By adjusting the pH of an EDTA
titration:
one type of metal ion (e.g. Fe
3+
) can
be titrated without interference from
others (e.g. Ca
2+
)
Minimum pH for Effective
Titration of Metal Ions
EDTA Titrations
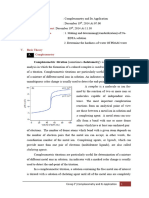
EDTA Titration Curves
1.) Titration Curve
The titration of a metal ion with EDTA is similar to the titration of a strong acid
(M
+
) with a weak base (EDTA)
The Titration Curve has three distinct regions:
- Before the equivalence point (excess M
n+
)
- At the equivalence point ([EDTA]=[M
n+
]
- After the equivalence point (excess EDTA)
- 4
Y
o
f
'
f
K K =
] [
+
=
n
M log pM
EDTA Titrations
EDTA Titration Curves
2.) Example
What is the value of [M
n+
] and pM for 50.0 ml of a 0.0500 M Mg
2+
solution
buffered at pH 10.00 and titrated with 0.0500 m EDTA when (a) 5.0 mL, (b)
50.0 mL and (c) 51.0 mL EDTA is added?
K
f
= 10
8.79
= 6.2x10
8
o
Y4-
at pH 10.0 = 0.30
( )( ) ( ) mL 00 . 50 V ) M 0500 . 0 ( mL 00 . 5 M 0500 . 0 ) mL ( V
e e
= =
mL EDTA at equivalence point:
mmol of EDTA
mmol of Mg
2+
EDTA Titrations
EDTA Titration Curves
2.) Example
(a) Before Equivalence Point ( 5.0 mL of EDTA)
Before the equivalence point, the [M
n+
] is equal to the concentration of excess
unreacted M
n+
. Dissociation of MY
n-4
is negligible.
] [
)] )( ( - ) )( [(
] [
L 0050 . 0 L 0500 . 0
L 0050 . 0 M EDTA 0500 . 0 L 0500 . 0 M Mg 0500 . 0
Mg
2
2
+
=
+
+
moles of Mg
2+
originally present
moles of EDTA added
Original volume
solution
Volume titrant
added
39 . 1 Mg log pMg M 0409 . 0 Mg
2 2 2
= = =
+ + +
] [ ] [
Dilution effect
EDTA Titrations
EDTA Titration Curves
2.) Example
(b) At Equivalence Point ( 50.0 mL of EDTA)
Virtually all of the metal ion is now in the form MgY
2-
) (
) (
) ( ] [
L 0500 . 0 L 0500 . 0
L 0500 . 0
M 0500 . 0 MgY
2
+
=
Original [M
n+
]
Original volume of
M
n+
solution
Original volume
solution
Volume titrant
added
Dilution effect
Moles Mg
+
moles MgY
2-
M 0250 . 0 MgY
2
=
] [
EDTA Titrations
EDTA Titration Curves
2.) Example
(b) At Equivalence Point ( 50.0 mL of EDTA)
The concentration of free Mg
2+
is then calculated as follows:
Initial Concentration (M) 0 0 0.0250
Final Concentration (M) x x 0.0250 - x
] ][ [
] ) [
EDTA Mg
EDTA ( Mg
K K
2
2 -
Y
f
'
4
f +
= =
o
) x )( x (
) x 0250 . 0 (
) 30 . 0 )( 10 2 . 6 (
8
=
Solve for x using the quadratic equation:
94 . 4 pMg 10 16 . 1 EDTA Mg x
2 5 2
= = = =
+ +
] [ ] [
EDTA Titrations
EDTA Titration Curves
2.) Example
(c) After the Equivalence Point ( 51.0 mL of EDTA)
Virtually all of the metal ion is now in the form MgY
2-
and there is excess,
unreacted EDTA. A small amount of free M
n+
exists in equilibrium with
MgY
4-
and EDTA.
) (
) )( (
] [
L 0510 . 0 L 0500 . 0
L 0010 . 0 M 0500 . 0
EDTA
+
=
Original [EDTA]
Volume excess
titrant
Original volume
solution
Volume titrant
added
Dilution effect
Excess moles EDTA
M 10 95 . 4 EDTA
4
= ] [
Calculate excess [EDTA]:
EDTA Titrations
EDTA Titration Curves
2.) Example
(c) After the Equivalence Point ( 51.0 mL of EDTA)
Calculate [MgY
2-
]:
) (
) (
) ( ] [
L 0510 . 0 L 0500 . 0
L 0500 . 0
M 0500 . 0 MgY
2
+
~
Original [M
n+
]
Original volume of
M
n+
solution
Original volume
solution
Volume titrant
added
Dilution effect
Moles Mg
+
moles MgY
2-
M 0248 . 0 MgY
2
~
] [
Only Difference
EDTA Titrations
EDTA Titration Curves
2.) Example
(c) After the Equivalence Point ( 51.0 mL of EDTA)
[Mg
2+-
] is given by the equilibrium expression using [EDTA] and [MgY
2-
]:
] ][ [
] ) [
EDTA Mg
EDTA ( Mg
K K
2
2 -
Y
f
'
4
f +
= =
o
) M 10 95 . 4 )( x (
) M 0248 . 0 (
) 30 . 0 )( 10 2 . 6 (
4
8
=
57 . 6 pMg 10 7 . 2 Mg x
2 7 2
= = =
+ +
] [
EDTA Titrations
EDTA Titration Curves
2.) Example
Final titration curve for 50.0 ml of 0.0500 M Mg
2+
with 0.0500 m EDTA at pH
10.00.
- Also shown is the titration of 50.0 mL of 0.0500 M Zn
2+
Note: the equivalence point is sharper for Zn
2+
vs. Mg
2+
. This is due to Zn
2+
having a larger
formation constant.
The completeness of these reactions is
dependent on o
Y4-
and correspondingly pH.
pH is an important factor in setting the completeness
and selectivity of an EDTA titration
EDTA Titrations
Auxiliary Complexing Agents
1.) Metal Hydroxide
In general, as pH increases a titration of a metal ion with EDTA will have a
higher K
f
.
- Larger change at the equivalence point.
Exception: If M
n+
reacts with OH
-
to form an insoluble metal hydroxide
Auxiliary Complexing Agents: a ligand can be added that complexes with M
n+
strong enough to prevent hydroxide formation.
- Ammonia, tartrate, citrate or triethanolamine
- Binds metal weaker than EDTA
f
Zn Y
' '
K K
2 4
f
+
= o o
n
n
2
2 1
M
] L [ ] L [ ] L [ 1
1
| | |
o
+ + +
=
Fraction of free metal ion (o
M
) depends on the
equilibrium constants (|) or cumulative formation
constants:
Use a new conditional formation constant that
incorporates the fraction of free metal:
EDTA Titrations
Auxiliary Complexing Agents
2.) Illustration:
Titration of Cu
+2
(CuSO
4
) with EDTA
Addition of Ammonia Buffer results in a dark blue solution
- Cu(II)-ammonia complex is formed
Addition of EDTA displaces ammonia with corresponding color change
CuSO
4
Cu-EDTA
Cu-ammonia
EDTA Titrations
Metal Ion Indicators
1.) Determination of EDTA Titration End Point
Four Methods:
1. Metal ion indicator
2. Mercury electrode
3. pH electrode
4. Ion-selective electrode
Metal Ion Indicator: a compound that changes color when it binds to a metal
ion
- Similar to pH indicator, which changes color with pH or as the compound
binds H
+
For an EDTA titration, the indicator must bind the metal ion less strongly than
EDTA
- Similar in concept to Auxiliary Complexing Agents
- Needs to release metal ion to EDTA
Potential
Measurements
(red) (colorless) (colorless)
(blue)
End Point indicated by a color
change from red to blue
EDTA Titrations
Metal Ion Indicators
2.) Illustration
Titration of Mg
2+
by EDTA
- Eriochrome Black T Indicator
Addition of EDTA
Before Near After
Equivalence point
EDTA Titrations
Metal Ion Indicators
3.) Common Metal Ion Indicators
Most are pH indicators and can only be used over a given pH range
EDTA Titrations
Metal Ion Indicators
3.) Common Metal Ion Indicators
Useful pH ranges
EDTA Titrations
EDTA Titration Techniques
1.) Almost all elements can be determined by EDTA titration
Needs to be present at sufficient concentrations
Extensive Literature where techniques are listed in:
1) G. Schwarzenbach and H. Flaschka, Complexometric Titrations,
Methuen:London, 1969.
2) H.A. Flaschka, EDTA Titrations, Pergamon Press:New York, 1959
3) C.N. Reilley, A.J. Bernard, Jr., and R. Puschel, In: L. Meites (ed.) Handbook of
Analytical Chemistry, McGraw-Hill:New York, 1963; pp. 3-76 to 3-234.
Some Common Techniques used in these titrations include:
a) Direct Titrations
b) Back Titrations
c) Displacement Titrations
d) Indirect Titrations
e) Masking Agents
EDTA Titrations
EDTA Titration Techniques
2.) Direct Titrations
Analyte is buffered to appropriate pH and is titrated directly with EDTA
An auxiliary complexing agent may be required to prevent precipitation of
metal hydroxide.
3.) Back Titrations
A known excess of EDTA is added to analyte
- Free EDTA left over after all metal ion is bound with EDTA
The remaining excess of EDTA is then titrated with a standard solution of a
second metal ion
Approach necessary if analyte:
- precipitates in the presence of EDTA
- Reacts slowly with EDTA
- Blocks the indicator
Second metal ion must not displace analyte from EDTA
>
4 4
Y
) ion metal ond (sec f
Y
) analyte ( f
K K o o
EDTA Titrations
EDTA Titration Techniques
4.) Displacement Titration
Used for some analytes that dont have satisfactory metal ion indicators
Analyte (M
n+
) is treated with excess Mg(EDTA)
2-
, causes release of Mg
2+
.
Amount of Mg
2+
released is then determined by titration with a standard EDTA
solution
- Concentration of released Mg
2+
equals [M
n+
]
+ +
>
4 2 4 n
Y ) Mg ( f Y ) M ( f
K K o o
Requires:
EDTA Titrations
EDTA Titration Techniques
5.) Indirect Titration
Used to determine anions that precipitate with metal ions
Anion is precipitated from solution by addition of excess metal ion
- ex. SO
4
2-
+ excess Ba
2+
- Precipitate is filtered & washed
Precipitate is then reacted with excess EDTA to bring the metal ion back into
solution
The excess EDTA is titrated with Mg
2+
solution
[Total EDTA] = [MY
n-4
] + [Y
4-
]
complex free
Known Titrate
determine
EDTA Titrations
EDTA Titration Techniques
6.) Masking Agents
A reagent added to prevent reaction of some metal ion with EDTA
Demasking: refers to the release of a metal ion from a masking agent
Al
3+
is not available to bind EDTA because of the complex with F
-
)) EDTA ( Al ( f
) AlF ( f
K K
3
6
>
Requires:
Das könnte Ihnen auch gefallen
- Psych PresurgicalDokument31 SeitenPsych Presurgicalriham ammar100% (1)
- Professional Letters GuideDokument10 SeitenProfessional Letters GuideRaees Swati100% (1)
- Business Report Writing SkillsDokument78 SeitenBusiness Report Writing Skillsssriram1990100% (13)
- KGB Complexometric TitrationDokument36 SeitenKGB Complexometric TitrationpharmaprvNoch keine Bewertungen
- Sheiko 13week Beginner ProgramDokument16 SeitenSheiko 13week Beginner ProgramAnders DahlNoch keine Bewertungen
- Production and Application of Bitumen EmulsionDokument3 SeitenProduction and Application of Bitumen Emulsionjignesh_panNoch keine Bewertungen
- Introduction To Polymer ChemistryDokument25 SeitenIntroduction To Polymer Chemistryjignesh_panNoch keine Bewertungen
- Scientology CommunicationDokument58 SeitenScientology CommunicationNicholas Edward Werner-Matavka100% (1)
- Impact of Technology On Our LivesDokument3 SeitenImpact of Technology On Our LivesKim ErandioNoch keine Bewertungen
- Complexometric Titrations - PPT 1Dokument26 SeitenComplexometric Titrations - PPT 1Khairi Mustafa Salem67% (3)
- Heat Cured ElastomersDokument40 SeitenHeat Cured ElastomerslberrierNoch keine Bewertungen
- Complexometric Titration: Complex (Coordination Compound)Dokument13 SeitenComplexometric Titration: Complex (Coordination Compound)Ben AbellaNoch keine Bewertungen
- Business Communication Self-Learning MaterialDokument287 SeitenBusiness Communication Self-Learning MaterialDeepak PatelNoch keine Bewertungen
- Complexmetric TitrationDokument17 SeitenComplexmetric TitrationAnonymous oC3F7cxlLH100% (1)
- Complexometric TitrationDokument25 SeitenComplexometric Titrationpumeananda100% (1)
- Complexometric EDTADokument36 SeitenComplexometric EDTANqobile Nqoerh MnguniNoch keine Bewertungen
- The Essential Handbook For Business WritingDokument62 SeitenThe Essential Handbook For Business WritingDandan JiaoNoch keine Bewertungen
- Marshall Method of Asphalt-Concrete Mix DesignDokument16 SeitenMarshall Method of Asphalt-Concrete Mix DesignfitrianiNoch keine Bewertungen
- Tittrations Based On Complex FormationDokument5 SeitenTittrations Based On Complex FormationNisaNoch keine Bewertungen
- EDTA Titrations: Metal Chelate ComplexesDokument35 SeitenEDTA Titrations: Metal Chelate ComplexesalphhabetaNoch keine Bewertungen
- 14) Coordination Chemistry PDFDokument28 Seiten14) Coordination Chemistry PDFBj Larracas100% (6)
- EdtaDokument13 SeitenEdtaChongZY100% (1)
- Estimation of Measurement Uncertainty For Electrical Conductivity in WaterDokument4 SeitenEstimation of Measurement Uncertainty For Electrical Conductivity in WaterMaruthi KNoch keine Bewertungen
- How To Improve Your Spoken EnglishDokument22 SeitenHow To Improve Your Spoken EnglishSyahamah FuzaiNoch keine Bewertungen
- How To Write A Cover LetterDokument19 SeitenHow To Write A Cover Letterjignesh_panNoch keine Bewertungen
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersVon EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersNoch keine Bewertungen
- EDTA Titration of Calcium and MagnesiumDokument3 SeitenEDTA Titration of Calcium and MagnesiumAnonymous NxpnI6jC67% (3)
- Calcium Chloride HandbookDokument28 SeitenCalcium Chloride Handbookwotthinun100% (1)
- Lewis Acid-Base Concept: Chapter 13 EDTA TitrationsDokument23 SeitenLewis Acid-Base Concept: Chapter 13 EDTA TitrationsEdin AbolenciaNoch keine Bewertungen
- Fluid Management in NicuDokument56 SeitenFluid Management in NicuG Venkatesh100% (2)
- Practice Makes Perfect in Chemistry: Compounds, Reactions and Moles with AnswersVon EverandPractice Makes Perfect in Chemistry: Compounds, Reactions and Moles with AnswersBewertung: 3 von 5 Sternen3/5 (2)
- Titrari EDTADokument35 SeitenTitrari EDTAAdrian BlidarNoch keine Bewertungen
- Complexometry: Pharmaceutical Analysis For Liquid and Semisolid PreparationDokument32 SeitenComplexometry: Pharmaceutical Analysis For Liquid and Semisolid PreparationaulianiNoch keine Bewertungen
- Chapter 12Dokument35 SeitenChapter 12Jolina PagulayanNoch keine Bewertungen
- EDTA Titrations: Metal Chelate ComplexesDokument35 SeitenEDTA Titrations: Metal Chelate ComplexesJenny LlanesNoch keine Bewertungen
- Apch231 EdtaDokument13 SeitenApch231 EdtaTan Ze KaiNoch keine Bewertungen
- Complexometric TitrationDokument64 SeitenComplexometric TitrationToyeba RahiNoch keine Bewertungen
- Complexometric and Precipitation TitrationsDokument10 SeitenComplexometric and Precipitation Titrationskhanny96100% (1)
- EDTADokument29 SeitenEDTA5P4RT4NII7Noch keine Bewertungen
- Chap 3. Complexation ReactionTitrationDokument51 SeitenChap 3. Complexation ReactionTitrationlesangg1406Noch keine Bewertungen
- General Chemistry IV: CHEM-224 Lecture 2: Titrimetric Methods of AnalysisDokument36 SeitenGeneral Chemistry IV: CHEM-224 Lecture 2: Titrimetric Methods of AnalysisGeorge chaupi NyondoNoch keine Bewertungen
- Complexation Titration Department of Physical Sciences & Technology University of Sabaragamuwa Srilanka (Dokument33 SeitenComplexation Titration Department of Physical Sciences & Technology University of Sabaragamuwa Srilanka (eswarNoch keine Bewertungen
- Complexometric Reactions and TitrationDokument6 SeitenComplexometric Reactions and TitrationAndre YosiNoch keine Bewertungen
- Complexometric Titration 1Dokument14 SeitenComplexometric Titration 1Girma Selale0% (1)
- 01 EDTA TitrationsDokument11 Seiten01 EDTA TitrationsAkhil P KumarNoch keine Bewertungen
- Complexationtitration KDBDokument31 SeitenComplexationtitration KDBKiranNoch keine Bewertungen
- Complex Formation Titration Sep08Dokument6 SeitenComplex Formation Titration Sep08Mark ReyesNoch keine Bewertungen
- Ch9 2021 2Dokument19 SeitenCh9 2021 2muhiafrancis975Noch keine Bewertungen
- 05 - Chapter 1 PDFDokument57 Seiten05 - Chapter 1 PDFravisankarNoch keine Bewertungen
- Chap 3. Complexation ReactionTitrationDokument45 SeitenChap 3. Complexation ReactionTitrationT.N NgânNoch keine Bewertungen
- Complexometry 7199 1Dokument9 SeitenComplexometry 7199 1Admiral General AmanNoch keine Bewertungen
- Analytical Chemistry Notes IvDokument6 SeitenAnalytical Chemistry Notes IvJabez MatigaNoch keine Bewertungen
- Chapter-9 ComplexmetricDokument21 SeitenChapter-9 Complexmetricmohamedzyad7717Noch keine Bewertungen
- Complexometry Determination Hardness ofDokument30 SeitenComplexometry Determination Hardness ofMichael CarloNoch keine Bewertungen
- Chap 3. Complexation ReactionTitrationDokument51 SeitenChap 3. Complexation ReactionTitrationKoasa NishikiNoch keine Bewertungen
- Phcm223 Lecture 7 Ss16 472Dokument26 SeitenPhcm223 Lecture 7 Ss16 472Jerson Fernando Arroyo BaldarragoNoch keine Bewertungen
- Complexometric TitrationDokument8 SeitenComplexometric TitrationYUNITA DWINoch keine Bewertungen
- EDTA Titration LabDokument16 SeitenEDTA Titration LabJosef Hilton67% (3)
- Practicum 8 Complexometric Titration Experiment Summary: ObjectivesDokument4 SeitenPracticum 8 Complexometric Titration Experiment Summary: ObjectivesMia RismaliaNoch keine Bewertungen
- Chapter 4Dokument29 SeitenChapter 4MD. Aminul IslamNoch keine Bewertungen
- Complexometry TitartaionDokument50 SeitenComplexometry TitartaionHaritNoch keine Bewertungen
- Course Name: Volumetric and Gravimetric Analytical Chemistry Course Code: 4022133-3Dokument26 SeitenCourse Name: Volumetric and Gravimetric Analytical Chemistry Course Code: 4022133-3faycalfaidiNoch keine Bewertungen
- Lecture 1. Complexometric TitrationDokument22 SeitenLecture 1. Complexometric TitrationMohamed Babiker SulimanNoch keine Bewertungen
- TYBSc Complexometric TitrationDokument88 SeitenTYBSc Complexometric Titrationmyebooks.pramodNoch keine Bewertungen
- Complexometric TitrationDokument4 SeitenComplexometric TitrationWendell Kim LlanetaNoch keine Bewertungen
- Complexometrictitration-Ppt Part III A 2Dokument21 SeitenComplexometrictitration-Ppt Part III A 2Anand NanavatyNoch keine Bewertungen
- Coordination Assignment 2Dokument6 SeitenCoordination Assignment 2sara noorNoch keine Bewertungen
- Ch13 Ch16 SuppDokument24 SeitenCh13 Ch16 SuppQuoc AnhNoch keine Bewertungen
- Complexometric Titration With EDTADokument8 SeitenComplexometric Titration With EDTAManP13100% (1)
- EDTA Titration CurveDokument14 SeitenEDTA Titration CurveM'maay WrtyNoch keine Bewertungen
- Section 08 Complex Formation Tit RationsDokument19 SeitenSection 08 Complex Formation Tit RationsHandugan Quinlog NoelNoch keine Bewertungen
- Chapter 24Dokument141 SeitenChapter 24teweNoch keine Bewertungen
- Ch13 Ch16 SuppDokument24 SeitenCh13 Ch16 SuppLai Le100% (1)
- Chapter 13 3811 EDTADokument35 SeitenChapter 13 3811 EDTAgaur1234Noch keine Bewertungen
- 08 - Chapter 1Dokument40 Seiten08 - Chapter 1Girmaye HaileNoch keine Bewertungen
- ComplexometryDokument73 SeitenComplexometryJerosha Ifthikar AhmedNoch keine Bewertungen
- Corrected Theoretical Basis of Analysis - Complexometric TitrationsDokument19 SeitenCorrected Theoretical Basis of Analysis - Complexometric TitrationsRajeswari RajiNoch keine Bewertungen
- Vibrational Spectra of Organometallics: Theoretical and Experimental DataVon EverandVibrational Spectra of Organometallics: Theoretical and Experimental DataNoch keine Bewertungen
- Chapter 12Dokument24 SeitenChapter 12Shashank TiwariNoch keine Bewertungen
- Road Emulsion AapplicationDokument4 SeitenRoad Emulsion AapplicationiGp2013Noch keine Bewertungen
- Module 9Dokument65 SeitenModule 9Catur SupriyantoNoch keine Bewertungen
- Eng Off SkillsDokument29 SeitenEng Off Skillsjignesh_panNoch keine Bewertungen
- SolutionsDokument4 SeitenSolutionsme_dayakarNoch keine Bewertungen
- Common Grammar Mistakes DFSDDokument3 SeitenCommon Grammar Mistakes DFSDbharath_mv7Noch keine Bewertungen
- Glass Capillary Viscometers-2006Dokument16 SeitenGlass Capillary Viscometers-2006etikafNoch keine Bewertungen
- Laboratory Evaluation of Warm Mix AsphaltDokument119 SeitenLaboratory Evaluation of Warm Mix Asphaltjignesh_panNoch keine Bewertungen
- Comskills ReferencesDokument16 SeitenComskills Referencesjignesh_panNoch keine Bewertungen
- Guide To PH MeasurementDokument52 SeitenGuide To PH Measurementletz911Noch keine Bewertungen
- Waste Mix AsphaltDokument68 SeitenWaste Mix Asphaltjignesh_panNoch keine Bewertungen
- Cover Letters CorrespondenceDokument12 SeitenCover Letters Correspondencejignesh_panNoch keine Bewertungen
- Asphalte GlossaryDokument15 SeitenAsphalte Glossaryjignesh_panNoch keine Bewertungen
- Module 9Dokument65 SeitenModule 9Catur SupriyantoNoch keine Bewertungen
- Aashto MethodsDokument0 SeitenAashto Methodsjignesh_panNoch keine Bewertungen
- 5S Tools PDFDokument7 Seiten5S Tools PDFjignesh_panNoch keine Bewertungen
- Liquid Calcium Chloride Data Sheet PDFDokument5 SeitenLiquid Calcium Chloride Data Sheet PDFjignesh_panNoch keine Bewertungen
- Admission Prspectus English 2021-2022Dokument9 SeitenAdmission Prspectus English 2021-2022A.B. SiNoch keine Bewertungen
- Research Proposal Sample OutlineDokument17 SeitenResearch Proposal Sample OutlineGuidance and Counseling OfficeNoch keine Bewertungen
- User Manual For Scanbox Ergo & Banquet Line: Ambient (Neutral), Hot and Active Cooling. Scanbox Meal Delivery CartsDokument8 SeitenUser Manual For Scanbox Ergo & Banquet Line: Ambient (Neutral), Hot and Active Cooling. Scanbox Meal Delivery CartsManunoghiNoch keine Bewertungen
- ABS CBN CorporationDokument16 SeitenABS CBN CorporationAlyssa BeatriceNoch keine Bewertungen
- Term Paper Gender RolesDokument5 SeitenTerm Paper Gender Rolesea8d1b6n100% (1)
- Ga2 27:6:23Dokument1 SeiteGa2 27:6:23john HuntNoch keine Bewertungen
- Ca Final Compiler Paper 5 Advanced Management Accounting PDFDokument432 SeitenCa Final Compiler Paper 5 Advanced Management Accounting PDFAnn SerratoNoch keine Bewertungen
- Former Rajya Sabha MP Ajay Sancheti Appeals Finance Minister To Create New Laws To Regulate Cryptocurrency MarketDokument3 SeitenFormer Rajya Sabha MP Ajay Sancheti Appeals Finance Minister To Create New Laws To Regulate Cryptocurrency MarketNation NextNoch keine Bewertungen
- IGCSE Religious Studies (Edexcel - 2009 - Be Careful Not To Choose The New' IGCSE)Dokument8 SeitenIGCSE Religious Studies (Edexcel - 2009 - Be Careful Not To Choose The New' IGCSE)Robbie TurnerNoch keine Bewertungen
- Bsee 36: Survey of English and American Literature Learning Material 2: Introduction To Literary Theories and CriticismDokument4 SeitenBsee 36: Survey of English and American Literature Learning Material 2: Introduction To Literary Theories and CriticismCarlosNorielCabanaNoch keine Bewertungen
- Service Instruction Selection of Suitable Operating Fluids For ROTAX Engine Type 916 I (Series), 915 I (Series), 912 I (Series), 912 and 914 (Series)Dokument15 SeitenService Instruction Selection of Suitable Operating Fluids For ROTAX Engine Type 916 I (Series), 915 I (Series), 912 I (Series), 912 and 914 (Series)Martin PilatiNoch keine Bewertungen
- CIVIL 3811 - Lecture Slides - Week 7Dokument58 SeitenCIVIL 3811 - Lecture Slides - Week 7hadaNoch keine Bewertungen
- Central Venous PressureDokument3 SeitenCentral Venous PressureHuy NguyễnNoch keine Bewertungen
- Education and Its LegitimacyDokument4 SeitenEducation and Its LegitimacySheila G. Dolipas100% (6)
- What Is E-CollaborationDokument7 SeitenWhat Is E-CollaborationToumba LimbreNoch keine Bewertungen
- ESUR Guidelines 10.0 Final VersionDokument46 SeitenESUR Guidelines 10.0 Final Versionkon shireNoch keine Bewertungen
- Frogs and ToadsDokument6 SeitenFrogs and ToadsFaris AlarshaniNoch keine Bewertungen
- Gunnar Fischer's Work On Ingmar Bergman's The Seventh Seal and Wild StrawberriesDokument6 SeitenGunnar Fischer's Work On Ingmar Bergman's The Seventh Seal and Wild StrawberriesSaso Dimoski100% (1)
- Prototyping: by DR Sampa Unnikrishnan Yateer Creative Solutions Reachus@Yateer - In, 8971442777Dokument70 SeitenPrototyping: by DR Sampa Unnikrishnan Yateer Creative Solutions Reachus@Yateer - In, 8971442777ShivashankarNoch keine Bewertungen
- Effect of Social Economic Factors On Profitability of Soya Bean in RwandaDokument7 SeitenEffect of Social Economic Factors On Profitability of Soya Bean in RwandaMarjery Fiona ReyesNoch keine Bewertungen
- Xiameter OFS-6020 Silane: Diaminofunctional Silane Features ApplicationsDokument2 SeitenXiameter OFS-6020 Silane: Diaminofunctional Silane Features ApplicationsDelovita GintingNoch keine Bewertungen
- Body LanguageDokument17 SeitenBody LanguageAR PiZaNoch keine Bewertungen
- Thesis Final 2 Number c1-c5Dokument167 SeitenThesis Final 2 Number c1-c5Kimverly DomaganNoch keine Bewertungen
- Sekonic L 758Dokument68 SeitenSekonic L 758mariosapereiraNoch keine Bewertungen