Beruflich Dokumente
Kultur Dokumente
Ethanol
Hochgeladen von
Marinca Ionut0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
102 Ansichten15 SeitenEthanol
Copyright
© © All Rights Reserved
Verfügbare Formate
PPTX, PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenEthanol
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PPTX, PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
102 Ansichten15 SeitenEthanol
Hochgeladen von
Marinca IonutEthanol
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PPTX, PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 15
Ethanol
Ethanol /nl/, also called ethyl
alcohol /l/, pure
alcohol, beverage alcohol,
or drinking alcohol, is
a volatile, flammable, colorless liquid
with the structural formula
CH
3
CH
2
OH, often abbreviated as
C
2
H
5
OH or C
2
H
6
O. Ethanol is
a psychoactive drug and is one of the
oldest recreational drugs still used by
humans. Ethanol can cause alcohol
intoxication when consumed. Best
known as the type of alcohol found
in alcoholic beverages, it is also used
in thermometers, as a solvent, and as
a fuel. In common usage, it is often
referred to simply
as alcohol or spirits.
The fermentation of sugar into ethanol is one of the earliest biotechnologies employed by
humans. The intoxicating effects of ethanol consumption have been known since ancient
times. Ethanol has been used by humans since prehistory as the intoxicating ingredient
of alcoholic beverages. Dried residue on 9,000-year-old pottery found in China suggests
that Neolithic people consumed alcoholic beverages.
In 1796, German-Russian chemist Johann Tobias Lowitz obtained pure ethanol by mixing
partially purified ethanol (the alcohol-water azeotrope) with an excess of anhydrous alkali
and then distilling the mixture over low heat. French chemist Antoine Lavoisier described
ethanol as a compound of carbon, hydrogen, and oxygen, and in 1807 Nicolas-Thodore
de Saussure determined ethanol's chemical formula.Fifty years later, Archibald Scott
Couper published the structural formula of ethanol. It was one of the first structural
formulas determined.
Ethanol was first prepared synthetically in 1825 by Michael Faraday. He found that
sulfuric acid could absorb large volumes of coal gas.He gave the resulting solution to
Henry Hennell, a British chemist, who found in 1826 that it contained "sulphovinic acid"
(ethyl hydrogen sulfate). In 1828, Hennell and the French chemist Georges-Simon
Srullas independently discovered that sulphovinic acid could be decomposed into
ethanol. Thus, in 1825 Faraday had unwittingly discovered that ethanol could be
produced from ethylene (a component of coal gas) by acid-catalyzed hydration, a process
similar to current industrial ethanol synthesis.
Ethanol was used as lamp fuel in the United States as early as 1840, but a tax levied on
industrial alcohol during the Civil War made this use uneconomical. The tax was repealed
in 1906.Use as an automotive fuel dates back to 1908, with the Ford Model T able to run
on gasoline or ethanol. It remains a common fuel for spirit lamps.
Alcoholic beverages
Ethanol is the principal psychoactive constituent in alcoholic beverages.
With depressant effects on the central nervous system, it has a complex
mode of action and affects multiple systems in the brain, most notably
increasing the activity of GABA receptors. Through positive allosteric
modulation, it enhances the activity of naturally produced GABA. Other
psychoactives such as benzodiazepines, barbiturates exert their effects by
binding to the same receptor complex, thus have similar CNS depressant
effects.
Alcoholic beverages vary considerably in ethanol content and in foodstuffs
they are produced from. Most alcoholic beverages can be broadly classified
as fermented beverages, beverages made by the action of yeast on sugary
foodstuffs, or distilled beverages, beverages whose preparation involves
concentrating the ethanol in fermented beverages by distillation. The
ethanol content of a beverage is usually measured in terms of the volume
fraction of ethanol in the beverage, expressed either as a percentage or
in alcoholic proof units.
Fermented
Fermented beverages can be
broadly classified by the foodstuff
they are fermented from. Beers are
made from cereal grains or
other starchy materials, wines and
ciders from fruit juices,
and meads from honey. Cultures
around the world have made
fermented beverages from
numerous other foodstuffs, and
local and national classifications
for various fermented beverages
abound.
Distilled
Distilled beverages are made by distilling
fermented beverages. Broad categories of
distilled beverages include whiskeys, distilled
from fermented cereal grains; brandies, distilled
from fermented fruit juices; and rum, distilled
fromfermented molasses or sugarcane juice. Vod
ka and similar neutral grain spirits can be
distilled from any fermented material (grain
and potatoes are most common); these spirits are
so thoroughly distilled that no tastes from the
particular starting material remain. Numerous
other spirits and liqueurs are prepared by
infusing flavors from fruits, herbs,
and spices into distilled spirits. A traditional
example is gin, which is created by
infusing juniper berries into a neutral grain
alcohol.
The ethanol content in alcoholic beverages can be increased by
means other than distillation. Applejack is traditionally made
by freeze distillation, by which water is frozen out of
fermented apple cider, leaving a more ethanol-rich liquid behind. Ice
beer (also known by the German term Eisbier) is also freeze-
distilled, with beer as the base beverage. Fortified wines are
prepared by adding brandy or some other distilled spirit to partially
fermented wine. This kills the yeast and conserves a portion of
the sugar in grape juice; such beverages are not only more ethanol-
rich but are often sweeter than other wines.
Alcoholic beverages are used in cooking for their flavors and
because alcohol dissolves hydrophobic flavor compounds.
Just as industrial ethanol is used as feedstock for the production of
industrial acetic acid, alcoholic beverages are made
into vinegar. Wine and cider vinegar are both named for their
respective source alcohols, whereas malt vinegar is derived from
beer.
Historical uses
Before the development of modern medicines, ethanol was used for
a variety of medical purposes. It has been known to be used as
a truth drug (as hinted at by the maxim "in vino veritas"), as
medicine for depression and as an anesthetic.
Ethanol was commonly used as fuel in
early bipropellant rocket (liquid propelled) vehicles, in conjunction
with an oxidizer such as liquid oxygen. The German V-2
rocket of World War II, credited with beginning the space age, used
ethanol, mixed with 25% of water to reduce the combustion
chamber temperature. The V-2's design team helped develop U.S.
rockets following World War II, including the ethanol-
fueled Redstone rocket which launched the first U.S.
satellite. Alcohols fell into general disuse as more efficient rocket
fuels were developed.
Effects on the central nervous
system
Ethanol is a central nervous
system depressant and has
significant psychoactive effects in
sublethal doses. Based on its
abilities to change the human
consciousness, ethanol is
considered a psychoactive
drug. Death from ethanol
consumption is possible when
blood alcohol level reaches 0.4%.
A blood level of 0.5% or more is
commonly fatal. Levels of even
less than 0.1% can
cause intoxication, with
unconsciousness often occurring
at 0.30.4%.
BAC
(g/L)
BAC
(% v/v)
Symptoms
[73]
0.5 0.05%
Euphoria, talkativeness,
relaxation
1 0.1 %
Central nervous system
depression, nausea, possible
vomiting, impaired motor and
sensory function, impaired
cognition
>1.4 >0.14% Decreased blood flow to brain
3 0.3%
Stupefaction, possible
unconsciousness
4 0.4% Possible death
>5.5 >0.55% Death
Ethanol acts in the central nervous system by binding to the GABA-
A receptor, increasing the effects of the inhibitory
neurotransmitter GABA (i.e., it is a positive allosteric modulator).
Prolonged heavy consumption of alcohol can cause significant
permanent damage to the brain and other organs. See Alcohol
consumption and health.
According to the US National Highway Traffic Safety
Administration, in 2002 about "41% of people fatally injured in
traffic crashes were in alcohol related crashes". The risk of a
fatal car accident increases exponentially with the level of alcohol in
the driver's blood.Most drunk driving laws governing the acceptable
levels in the blood while driving or operating heavy machinery set
typical upper limits of blood alcohol content (BAC) between 0.02%
and 0.08%.
Discontinuing consumption of alcohol after several years of heavy
drinking can also be fatal. Alcohol withdrawal can cause anxiety,
autonomic dysfunction, seizures, and hallucinations. Delirium
tremens is a condition that requires people with a long history of
heavy drinking to undertake an alcohol detoxification regimen.
Alcohol and digestion
A part of ethyl alcohol is
hydrophobic. This hydrophobic or
lipophilic end can diffuse across cells
that line the stomach wall. In fact,
alcohol is one of the rare substances
that can be absorbed in the stomach.
Most food substances are absorbed in
the small intestine. However, even
though alcohol can be absorbed in the
stomach, it is mostly absorbed in the
small intestine because the small
intestine has a large surface area that
promotes absorption. Once alcohol is
absorbed in the small intestine, it
delays the release of stomach contents
from emptying into the small
intestine. Thus, alcohol can delay the
rate of absorption of nutrients. After
absorption, alcohol reaches the liver
where it is metabolized.
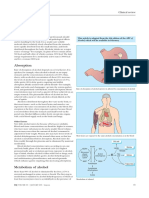
How Breathalyzers work:
Alcohol that is not processed by the liver goes to the
heart. The liver can process only a certain amount of
alcohol per unit time. Thus, when a person drinks too
much alcohol, more alcohol can reach the heart. In the
heart, alcohol reduces the force of heart contractions.
Consequently, the heart will pump less blood, lowering
overall body blood pressure. Also, blood that reaches
the heart goes to the lungs to replenish blood's oxygen
concentration. It is at this stage that a person can
breathe out traces of alcohol. This is the underlying
principle of the alcohol breath testing (or breathalyzers)
to determine if a driver has been drinking and driving.
From the lungs, blood returns to the heart and will be distributed
throughout the body. Interestingly, alcohol increases levels of high-
density lipoproteins(HDLs), which carry cholesterol. Alcohol is
known to make blood less likely to clot, reducing risk of heart attack
and stroke. This could be the reason why alcohol could produce
health benefits when consumed in moderate amounts. Also, alcohol
dilates blood vessels. Consequently, a person will feel warmer, and
their face turns flush and pink.
Why people lose their sense of balance after drinking alcohol:
When alcohol reaches the brain, it has the ability to delay signals
that are sent between nerve cells that control balance, thinking and
movement.
Why people frequently urinate after drinking alcohol:
Moreover, alcohol can affect the brain's ability to produce
antidiuretic hormones. These hormones are responsible for
controlling the amount of urine that is produced. Alcohol prevents
the body from reabsorbing water, and consequently a person who
recently drank alcohol will urinate frequently.
Other uses
Motor fuel
The largest single use of ethanol is as a
motor fuel and fuel additive. More than any other major
country, Brazil relies on ethanol as a motor
fuel. Gasoline sold in Brazil contains at least 25%
anhydrous ethanol. Hydrous ethanol (about 95%
ethanol and 5% water) can be used as fuel in more than
90% of new cars sold in the country. Brazilian ethanol
is produced from sugar cane and noted for high carbon
sequestration. The US uses Gasohol (max 10% ethanol)
and E85 (85% ethanol) ethanol/gasoline mixtures.
Household heating
An example of a bio-ethanol fire in the form of a traditional
fireplace, using fire-proof ceramic simulated wood logs for effect.
Ethanol fuels flue-less, real flame fireplaces. Ethanol is kept in a
burner containing a wick such as glass wool, a safety shield to
reduce the chances of accidents and an extinguisher such as a plate
or shutter to cut off oxygen.
It provides almost the same visual benefits of a real flame log or
coal fire without the need to vent the fumes via a flue as ethanol
produces very little hazardous carbon monoxide, and little or no
noticeable scent. It does emit carbon dioxide and requires oxygen.
Therefore, external ventilation of the room containing the fire is
needed to ensure safe operation.
An additional benefit is that, unlike a flue based fireplace, 100% of
the heat energy produced enters the room. This serves to offset some
of the heat loss from an external air vent, as well as offset the
relatively high cost of the fuel compared to other forms of heating.
Feedstock
Ethanol is an important industrial ingredient and has
widespread use as a base chemical for other organic
compounds. These include ethyl halides, ethyl esters,
diethyl ether, acetic acid, ethyl amines, and, to a lesser
extent, butadiene.
Antiseptic
Ethanol is used in medical wipes and in most common
antibacterial hand sanitizer gels at a concentration of about
62% v/v as an antiseptic. Ethanol kills organisms
by denaturing their proteins and dissolving their lipids and
is effective against most bacteria and fungi, and
many viruses, but is ineffective against bacterial spores.
Solvent
Ethanol is miscible with water and is a good general
purpose solvent. It is found in paints, tinctures, markers, and
personal care products such as perfumes and deodorants.
Das könnte Ihnen auch gefallen
- Ujjval ChemistryDokument9 SeitenUjjval ChemistryNilesh DamorNoch keine Bewertungen
- Quit Alcohol like a Woman: How to Quit Alcohol Addiction, Stop Drinking, and Get Back Control of Your LifeVon EverandQuit Alcohol like a Woman: How to Quit Alcohol Addiction, Stop Drinking, and Get Back Control of Your LifeNoch keine Bewertungen
- Ujjval Che FinalDokument9 SeitenUjjval Che FinalBhargav JadavNoch keine Bewertungen
- Science AlcoholDokument10 SeitenScience AlcoholRic Andre SaludoNoch keine Bewertungen
- Awat Uses of Alcohol PDFDokument7 SeitenAwat Uses of Alcohol PDFAwat MuhammadNoch keine Bewertungen
- AlcoholDokument44 SeitenAlcoholJOHN FRITS GERARD MOMBAYNoch keine Bewertungen
- Quarter 4 Health Alcohol 1Dokument46 SeitenQuarter 4 Health Alcohol 1Clarette PacumioNoch keine Bewertungen
- 5.1.distillation ComplexDokument5 Seiten5.1.distillation ComplexSara SilesNoch keine Bewertungen
- BeveragesDokument3 SeitenBeveragesDheeraj SharmaNoch keine Bewertungen
- Alcohol in Islamic PerspectiveDokument44 SeitenAlcohol in Islamic PerspectiveannisyifaNoch keine Bewertungen
- Distillation - The Science of DistillationDokument3 SeitenDistillation - The Science of DistillationFoo Cheok HwaNoch keine Bewertungen
- Beverage PPT For 4th Year 2022Dokument225 SeitenBeverage PPT For 4th Year 2022RebkaNoch keine Bewertungen
- Alcohol 2Dokument19 SeitenAlcohol 2Febz CanutabNoch keine Bewertungen
- Complete PaperDokument24 SeitenComplete PaperirhammunazatNoch keine Bewertungen
- Alcohol PDFDokument5 SeitenAlcohol PDFJack KowmanNoch keine Bewertungen
- Properties: Alcoholic BeveragesDokument3 SeitenProperties: Alcoholic BeveragesPrinston Del CampoNoch keine Bewertungen
- Topic 4Dokument32 SeitenTopic 4Geraldine ValenciaNoch keine Bewertungen
- Describe The Characters On The Video What Could Be The Reason Behind This Behavior?Dokument26 SeitenDescribe The Characters On The Video What Could Be The Reason Behind This Behavior?Junadel RosalesNoch keine Bewertungen
- Process OutlineDokument39 SeitenProcess Outlinezekariyasa3Noch keine Bewertungen
- Alcoholic Beverage - Wikipedia, The Free EncyclopediaDokument13 SeitenAlcoholic Beverage - Wikipedia, The Free EncyclopediaravidraoNoch keine Bewertungen
- Alcoholism As A Vice ControlDokument12 SeitenAlcoholism As A Vice ControlCristine AvancenaNoch keine Bewertungen
- B. Physical Properties of AlcoholsDokument4 SeitenB. Physical Properties of AlcoholsAnjo Pasiolco CanicosaNoch keine Bewertungen
- Beverage Analysis in Forensic and Liquor SamplesDokument6 SeitenBeverage Analysis in Forensic and Liquor SamplesSachin ashokNoch keine Bewertungen
- Industrial Alcohol Production ProcessDokument24 SeitenIndustrial Alcohol Production Processzekariyasa3Noch keine Bewertungen
- The Uses of Alcohol: MedicineDokument6 SeitenThe Uses of Alcohol: MedicineNox sMilesNoch keine Bewertungen
- Beverage What Is Beverage? Drink BeverageDokument2 SeitenBeverage What Is Beverage? Drink BeverageriyacomputerNoch keine Bewertungen
- As Chemistry Notes AlcoholDokument13 SeitenAs Chemistry Notes AlcoholTai PanNoch keine Bewertungen
- SEM (CC3) ToxicologyDokument5 SeitenSEM (CC3) ToxicologyBea EvangelistaNoch keine Bewertungen
- Alcohol MetabolismDokument12 SeitenAlcohol Metabolismjames100% (1)
- Article On Alcoholic BeverageDokument128 SeitenArticle On Alcoholic BeverageArjohnaNoch keine Bewertungen
- Alcohol Pharmacology Siju Prakash Assistant ProfessorDokument29 SeitenAlcohol Pharmacology Siju Prakash Assistant ProfessorFuckyouNoch keine Bewertungen
- MODULE 4 Organic ChemDokument11 SeitenMODULE 4 Organic Chemangelo aquinoNoch keine Bewertungen
- Alcohol: in Ancient Time, Alcohol Has Been Part of Rituals, Medicines, Celebration/ CeremoniesDokument4 SeitenAlcohol: in Ancient Time, Alcohol Has Been Part of Rituals, Medicines, Celebration/ CeremoniesJhestonie P. PacisNoch keine Bewertungen
- 25 Words EthanolDokument4 Seiten25 Words EthanolHaleem KhanNoch keine Bewertungen
- What Is Ethanol?Dokument3 SeitenWhat Is Ethanol?Tamesh JodhanNoch keine Bewertungen
- L3 Responsible Alcohol Service and Social Awareness 2023-09-13 03 - 54 - 40Dokument50 SeitenL3 Responsible Alcohol Service and Social Awareness 2023-09-13 03 - 54 - 40Dickson ChanNoch keine Bewertungen
- College Municipal 3ème 3Dokument2 SeitenCollege Municipal 3ème 3Guillaume Olivier DjadouNoch keine Bewertungen
- 25 Words EthanolDokument4 Seiten25 Words EthanolMattrosen TimanNoch keine Bewertungen
- Alcohol IntroductionDokument2 SeitenAlcohol IntroductionPrerana PawarNoch keine Bewertungen
- Org Chem Lec Module 8 Alcohols and PhenolsDokument13 SeitenOrg Chem Lec Module 8 Alcohols and PhenolsAlfred.Noch keine Bewertungen
- GPT 1Dokument15 SeitenGPT 1Mekonnen AssefaNoch keine Bewertungen
- Alcohol Chemistry: Alcoholic Drinks (Ethanol) Solvents FuelsDokument17 SeitenAlcohol Chemistry: Alcoholic Drinks (Ethanol) Solvents FuelsSubhash DhungelNoch keine Bewertungen
- Alcohol AbuseDokument13 SeitenAlcohol AbuseSandy SandyNoch keine Bewertungen
- The Chemistry of VodkaDokument1 SeiteThe Chemistry of VodkastanculeanuNoch keine Bewertungen
- Ethanol: Done By: Shalenne Bahadur Subject: Chemistry Class: U6SDokument10 SeitenEthanol: Done By: Shalenne Bahadur Subject: Chemistry Class: U6SVarune kingNoch keine Bewertungen
- Applications of Alcohols REPORTDokument16 SeitenApplications of Alcohols REPORTalxxndraromeroNoch keine Bewertungen
- By: Sofía Escobar, Daniela Camacho and Adriana SalesDokument11 SeitenBy: Sofía Escobar, Daniela Camacho and Adriana SalesMariana Escobar DangondNoch keine Bewertungen
- AnalysisDokument3 SeitenAnalysisMarigold RamirezNoch keine Bewertungen
- Alcohol: "To Alcohol! The Cause Of... and Solution To... All of Life's Problems"Dokument9 SeitenAlcohol: "To Alcohol! The Cause Of... and Solution To... All of Life's Problems"Ma ElNoch keine Bewertungen
- Chemistry Investigatory ProjectDokument11 SeitenChemistry Investigatory ProjectDeepa SinghNoch keine Bewertungen
- Alcohol in The BodyDokument3 SeitenAlcohol in The BodyMarius StancuNoch keine Bewertungen
- Alcohol Devashish-1Dokument3 SeitenAlcohol Devashish-1devashish chouhanNoch keine Bewertungen
- ChatGPT Bartenders ManualDokument35 SeitenChatGPT Bartenders ManualAbulafalat13Noch keine Bewertungen
- Andaloc - BMT142 A1 Ass.2Dokument2 SeitenAndaloc - BMT142 A1 Ass.2Jeven AndalocNoch keine Bewertungen
- Chemistry Module 4&5Dokument11 SeitenChemistry Module 4&5angelo aquinoNoch keine Bewertungen
- Ethanol:: Do Not Confuse Ethanol With Methanol Which Will Probably Make You Go Blind or Die If You Drink ItDokument4 SeitenEthanol:: Do Not Confuse Ethanol With Methanol Which Will Probably Make You Go Blind or Die If You Drink ItRajib SarkarNoch keine Bewertungen
- AlcoholDokument23 SeitenAlcoholDr.j.sakthi Varshini 103Noch keine Bewertungen
- The Chemistry of EthanolDokument21 SeitenThe Chemistry of EthanolHarvey Obispo AceritNoch keine Bewertungen
- Etanol Vs MetanolDokument11 SeitenEtanol Vs MetanolSri WulanNoch keine Bewertungen
- EthanolDokument15 SeitenEthanolMarinca IonutNoch keine Bewertungen
- Penumbra Overture - Manual PDFDokument11 SeitenPenumbra Overture - Manual PDFMarinca IonutNoch keine Bewertungen
- Penumbra Requiem - WalkthroughDokument9 SeitenPenumbra Requiem - WalkthroughMarinca IonutNoch keine Bewertungen
- Penumbra Black Plague - WalkthroughDokument7 SeitenPenumbra Black Plague - WalkthroughMarinca IonutNoch keine Bewertungen
- Nothing Important Here EitherDokument1 SeiteNothing Important Here EitherMarinca IonutNoch keine Bewertungen
- Nothing Important HereDokument1 SeiteNothing Important HereMarinca IonutNoch keine Bewertungen