Beruflich Dokumente
Kultur Dokumente
Thermodynamic Cycles
Hochgeladen von
Rudra PratapCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Thermodynamic Cycles
Hochgeladen von
Rudra PratapCopyright:
Verfügbare Formate
Thermodynamic Cycles
Chapters 8, 9 and 10
For the rest of the semester..
Look at different cycles that approximate real
processes
You can categorize these processes several
different ways
Power Cycles vs Refrigeration
Gas vs Vapor
Closed vs open
Internal Combustion vs External Combustion
Power Cycles
Subject of Chapters 8 and 9
Otto Cycle
Spark Ignition
Diesel Cycle
Brayton Cycle
Gas Turbine
Rankine Cycle
Vapor
Real vs. Ideal
Thermal Efficiency
q
th
net
in
W
Q
=
These are all heat engines. They convert heat
to work, so the efficiency is:
Ideal Cycles
Well be using ideal cycles to analyze real
systems, so lets start with the only ideal cycle
weve studied thus far
Carnot Cycle
Ts diagram
T
S
1 2
1-2 Isothermal Heat
Addition
3
2-3 Isentropic
Expansion
4
3-4 Isothermal
Heat Rejection
4-1 Isentropic
Compression
Q=T dS
}
Pv Diagram
P
v
4-1 Isentropic
Compression
1
2
1-2 Isothermal Heat
Addition
3
2-3 Isentropic
Expansion
3-4 Isothermal
Heat Rejection
4
W=P dV
}
And
Since it is a cycle
Q-W=0
Q=W
In addition, we know that the efficiency for a
Carnot Cycle is:
q
th Carnot
L
H
T
T
,
= 1
W
Q
W
net
= Q
net
Carnot Cycle is not a good model for
most things
For example
Internal combustion engine
Gas turbine
We need to develop a new model, that is still
ideal
Air-Standard Assumptions
Air continuously circulates in a closed loop and
behaves as an ideal gas
All the processes are internally reversible
Combustion is replaced by a heat-addition
process from the outside
Heat rejection replaces the exhaust process
Cold Air Standard Assumptions
Also assume a constant value for C
p
, evaluated
at room temperature
Terminology for Reciprocating
Devices
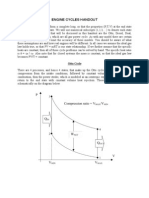
Compression Ratio
r
V
V
V
V
BDC
TDC
= =
max
min
Mean Effective Pressure
}
=
2
1
PdV W
V P W A =
v
v
1-2 Isentropic Compression
2-3 Constant Volume Heat Addition
3-4 Isentropic Expansion
4-1 Constant Volume Heat Rejection
Thermal Efficiency of the Otto Cycle
q
th
net
in
net
in
in out
in
out
in
W
Q
Q
Q
Q Q
Q
Q
Q
= = =
= 1
Apply First Law Closed System to Process 2-3,
V = Constant
}
= + = + =
A =
3
2
23 , 23 , 23 ,
23 23 , 23 ,
0 0 PdV W W W
U W Q
b other net
net net
Q U
Q Q mC T T
net
net in v
,
,
( )
23 23
23 3 2
=
= =
A
Apply First Law Closed System to Process 4-1,
V = Constant
0 0
1
4
41 , 41 , 41 ,
41 41 , 41 ,
= + = + =
A =
}
PdV W W W
U W Q
b other net
net net
Q U
Q Q mC T T
Q mC T T mC T T
net
net out v
out v v
,
,
( )
( ) ( )
41 41
41 1 4
1 4 4 1
=
= =
= =
A
q
th Otto
out
in
v
v
Q
Q
mC T T
mC T T
,
( )
( )
=
=
1
1
4 1
3 2
q
th Otto
T T
T T
T T T
T T T
,
( )
( )
( / )
( / )
=
1
1
1
1
4 1
3 2
1 4 1
2 3 2
Recall processes 1-2 and 3-4 are isentropic, so
1
3
4
4
3
1
2
1
1
2
T
and
|
|
.
|
\
|
=
|
|
.
|
\
|
=
k k
v
v T
v
v
T
T
But..
V
3
= V
2
and V
4
= V
1
T
T
T
T
or
T
T
T
T
2
1
3
4
4
1
3
2
=
=
T
T
T
T
or
T
T
T
T
2
1
3
4
4
1
3
2
=
=
q
th Otto
T T
T T
T T T
T T T
,
( )
( )
( / )
( / )
=
1
1
1
1
4 1
3 2
1 4 1
2 3 2
1
q
th Otto
T
T
,
= 1
1
2
Is this the same as the Carnot
efficiency?
NO!!
Efficiency of the Otto Cycle vs Carnot
Cycle
There are only two temperatures in the Carnot
cycle
Heat is added at T
H
Heat is rejected at T
L
There are four temperatures in the Otto cycle!!
Heat is added over a range of temperatures
Heat is rejected over a range of temperatures
1
1
1
2
2
1
1
=
|
|
.
|
\
|
=
k
k
r V
V
T
T
Since process 1-2 is isentropic,
q
th Otto
k
r
,
=
1
1
1
q
th Otto
T
T
,
= 1
1
2
Increasing Compression Ratio
Increases the Efficiency
Typical
Compression
Ratios for
Gasoline Engines
Why not use higher compression
Ratios?
Premature Ignition
Causes Knock
Reduces the Efficiency
Hard on the Engine
Das könnte Ihnen auch gefallen
- Gas Power Cycles Study Guide in Powerpoint: To AccompanyDokument68 SeitenGas Power Cycles Study Guide in Powerpoint: To AccompanyManjunatha TnNoch keine Bewertungen
- Chapter 8: Gas Power Cycles: TH Net inDokument50 SeitenChapter 8: Gas Power Cycles: TH Net inSures RezNoch keine Bewertungen
- Thermodynamic CyclesDokument32 SeitenThermodynamic CyclessunitbhaumikNoch keine Bewertungen
- Thermodynamic Cycle PresentationDokument29 SeitenThermodynamic Cycle PresentationAnonymous Oh1pxYX30% (1)
- Gas Power Cycles Study Guide in Powerpoint: To AccompanyDokument68 SeitenGas Power Cycles Study Guide in Powerpoint: To AccompanyAbraham HutomoNoch keine Bewertungen
- Ch20 Young Freedman2Dokument28 SeitenCh20 Young Freedman2Andrew MerrillNoch keine Bewertungen
- Web6 Combuction SystemDokument11 SeitenWeb6 Combuction SystemeswarbobbyNoch keine Bewertungen
- Stirling Heat Engine - Internal Combustion Engine (Otto Cycle) - Diesel Engine - Steam Engine (Rankine Cycle) - Kitchen RefrigeratorDokument11 SeitenStirling Heat Engine - Internal Combustion Engine (Otto Cycle) - Diesel Engine - Steam Engine (Rankine Cycle) - Kitchen RefrigeratormarzinusNoch keine Bewertungen
- IC Engines 2012 Edition Theory & QuestionsDokument178 SeitenIC Engines 2012 Edition Theory & Questionskumarrohit91Noch keine Bewertungen
- Otto CycleDokument28 SeitenOtto CycleNazeeh Abdulrhman AlbokaryNoch keine Bewertungen
- Term Odin A MikaDokument27 SeitenTerm Odin A MikaAnonymous GTCOMvNoch keine Bewertungen
- Chap5airstandardcycle2010 130703012738 Phpapp02Dokument54 SeitenChap5airstandardcycle2010 130703012738 Phpapp02Abdelkader Faklani DouNoch keine Bewertungen
- Gas Power CyclesDokument140 SeitenGas Power CyclesMohammed AlsirajNoch keine Bewertungen
- Second Law of Thermodynamics: T T Q QDokument10 SeitenSecond Law of Thermodynamics: T T Q Qnellai kumarNoch keine Bewertungen
- Gas Power Cycles Sivakumar.E VITDokument47 SeitenGas Power Cycles Sivakumar.E VITmohan govindasamyNoch keine Bewertungen
- Principle of TurbomachineryDokument159 SeitenPrinciple of TurbomachinerySharath ChandraNoch keine Bewertungen
- Module 5Dokument17 SeitenModule 5captainhassNoch keine Bewertungen
- Vapour Power CycleDokument12 SeitenVapour Power Cyclelakshmikanth97Noch keine Bewertungen
- S (Hot Reservoir) - Q S (Cold Reservoir) + - Q: - / T - / T S (Engine) 0 (Cyclic Process)Dokument51 SeitenS (Hot Reservoir) - Q S (Cold Reservoir) + - Q: - / T - / T S (Engine) 0 (Cyclic Process)കൂട്ടുകാരിയെ സ്നേഹിച്ച കൂട്ടുകാരൻNoch keine Bewertungen
- Ic Engine Cycles 1Dokument85 SeitenIc Engine Cycles 1jhpandiNoch keine Bewertungen
- Assignment 1 SolnDokument13 SeitenAssignment 1 SolnAlbert_LZK100% (4)
- Internal Combustion Engines: LecturDokument32 SeitenInternal Combustion Engines: LecturPuneet GargNoch keine Bewertungen
- Advanced Thermodynamics: Volumetric Properties of Pure FluidsDokument36 SeitenAdvanced Thermodynamics: Volumetric Properties of Pure FluidsArunodhayam NatarajanNoch keine Bewertungen
- Otto Diesel Dual Ideal Cycle - PPT (Compatibility Mode)Dokument16 SeitenOtto Diesel Dual Ideal Cycle - PPT (Compatibility Mode)Danang Wahdiat Aulia Ishaq0% (1)
- L27 - The Brayton CycleDokument12 SeitenL27 - The Brayton CycleHaliunaa BatboldNoch keine Bewertungen
- Unit - 2 Vapour Power Cycle - Theory - NotesDokument14 SeitenUnit - 2 Vapour Power Cycle - Theory - Notes1DS19ME136-Shivam KumarNoch keine Bewertungen
- Engineering Council Certificate Level Thermodynamic, Fluid and Process Engineering C106 Tutorial 5 - Ideal Engine CyclesDokument14 SeitenEngineering Council Certificate Level Thermodynamic, Fluid and Process Engineering C106 Tutorial 5 - Ideal Engine CyclesDipeshNoch keine Bewertungen
- Gas Cycles Otto, Diesel, Dual CyclesDokument43 SeitenGas Cycles Otto, Diesel, Dual Cyclesprasad5034100% (1)
- Gas Power CyclesDokument47 SeitenGas Power CyclesstansilawNoch keine Bewertungen
- Handbook Termodinamika, Komang SuardikaDokument22 SeitenHandbook Termodinamika, Komang SuardikaKomang SuardikaNoch keine Bewertungen
- Lecture Notes OnDokument200 SeitenLecture Notes Onananth k r100% (3)
- Ideal Rankine CycleDokument27 SeitenIdeal Rankine Cycleslv_prasaadNoch keine Bewertungen
- Ideal Engine CycleDokument20 SeitenIdeal Engine CycleMulugeta WoldeNoch keine Bewertungen
- Mechanical Engineering Thermodynamics II - Lecture 03 - 27 SepDokument25 SeitenMechanical Engineering Thermodynamics II - Lecture 03 - 27 SepThineshraaj Naidu Jaya RamanNoch keine Bewertungen
- School of Physics and Astronomy: File Topic07 PDFDokument3 SeitenSchool of Physics and Astronomy: File Topic07 PDFEbert AroneNoch keine Bewertungen
- Thermo CyclesDokument3 SeitenThermo CyclesRhandi MuliaNoch keine Bewertungen
- 5 Carnot & 3 Processes CyclesDokument27 Seiten5 Carnot & 3 Processes CyclesSarTomNoch keine Bewertungen
- Cyclic Process Second Law EnginesDokument18 SeitenCyclic Process Second Law EnginesM Khaidiz RafiNoch keine Bewertungen
- Thermodynamics 1 - Energy Analysis of Closed SystemsDokument26 SeitenThermodynamics 1 - Energy Analysis of Closed SystemsFlorasaurus17100% (2)
- Chap5 4Dokument8 SeitenChap5 4Christopher EvanNoch keine Bewertungen
- School of Physics and AstronomyDokument4 SeitenSchool of Physics and AstronomyzjnsrbtNoch keine Bewertungen
- ⎟⎟ ⎠ ⎞ ⎜⎜ ⎝ ⎛ − − = T T 1 T T 1 1 η: www.freestudy.co.ukDokument1 Seite⎟⎟ ⎠ ⎞ ⎜⎜ ⎝ ⎛ − − = T T 1 T T 1 1 η: www.freestudy.co.ukcataiceNoch keine Bewertungen
- 1st Law of ThermodynamicsDokument95 Seiten1st Law of ThermodynamicsMARUMO_LEVYNoch keine Bewertungen
- CH 02Dokument20 SeitenCH 02Pauline Nguyen100% (1)
- Theoretical CyclesDokument49 SeitenTheoretical CyclesMariaEzzaSyUyNoch keine Bewertungen
- Air Standard CyclesDokument28 SeitenAir Standard CyclesAditya Krishnakumar100% (1)
- Thermal EngineeringDokument118 SeitenThermal EngineeringSuresh RajuNoch keine Bewertungen
- Air Standard Cycles - BasicsDokument34 SeitenAir Standard Cycles - Basicsrazvan66m100% (1)
- Deal With Systems That Produce Power in Which The Working Fluid Remains A Gas Throughout The Cycle, I.e., No Change in PhaseDokument22 SeitenDeal With Systems That Produce Power in Which The Working Fluid Remains A Gas Throughout The Cycle, I.e., No Change in PhasePushpa Mohan RajNoch keine Bewertungen
- Lecture 3 Air CyclesDokument32 SeitenLecture 3 Air CyclesMemo KhalidNoch keine Bewertungen
- Lecture 01Dokument30 SeitenLecture 01Diane ClaireNoch keine Bewertungen
- Thermodynamic Cycles: Wed. Dec. 1, 2004Dokument26 SeitenThermodynamic Cycles: Wed. Dec. 1, 2004eviroyerNoch keine Bewertungen
- Dual CycleDokument3 SeitenDual CycleMohammad Faraz AkhterNoch keine Bewertungen
- 3 Ideal Models of Engine Processes and CyclesDokument58 Seiten3 Ideal Models of Engine Processes and Cyclesdinosaur x-drakeNoch keine Bewertungen
- Thermodynamic ProcessesDokument5 SeitenThermodynamic ProcessesKarthick RamNoch keine Bewertungen
- PNEUMATICS AND AIR CIRCUITS UNDERSTANDING THE CASCADE VALVE AND PLC UNDERSTANDINGVon EverandPNEUMATICS AND AIR CIRCUITS UNDERSTANDING THE CASCADE VALVE AND PLC UNDERSTANDINGNoch keine Bewertungen
- Working Guide to Vapor-Liquid Phase Equilibria CalculationsVon EverandWorking Guide to Vapor-Liquid Phase Equilibria CalculationsBewertung: 5 von 5 Sternen5/5 (1)
- Cambridge Revision Topic 11.3 and 21.1 With AnswersDokument13 SeitenCambridge Revision Topic 11.3 and 21.1 With AnswersMarin PesicNoch keine Bewertungen
- ABB Raw Mix Preparation PDFDokument8 SeitenABB Raw Mix Preparation PDFrudye kardun100% (1)
- Terrazo Concrete: Characteristics of TerrazzoDokument8 SeitenTerrazo Concrete: Characteristics of Terrazzodanishali1090Noch keine Bewertungen
- Weight Fixed Cone ValveDokument9 SeitenWeight Fixed Cone ValveJohn TLNoch keine Bewertungen
- Sodium Hexameta Phosphate e CHB 038Dokument1 SeiteSodium Hexameta Phosphate e CHB 038Wasif KarimNoch keine Bewertungen
- Alchemy at The Crowning of NatureDokument30 SeitenAlchemy at The Crowning of NatureMano DasruthiNoch keine Bewertungen
- Final - Basic Lasting TechnologyDokument137 SeitenFinal - Basic Lasting TechnologySumit Kumar Singh100% (1)
- Fluorescence N PhosphorescenceDokument14 SeitenFluorescence N Phosphorescenceanon_543130923Noch keine Bewertungen
- Alpha s708 - TdsDokument2 SeitenAlpha s708 - TdsMahmoud Moustafa ElnegihiNoch keine Bewertungen
- Selective PrecipitationDokument6 SeitenSelective PrecipitationEdcademiaNoch keine Bewertungen
- As 4964-2004 Method For The Qualitative Identification of Asbestos in Bulk SamplesDokument7 SeitenAs 4964-2004 Method For The Qualitative Identification of Asbestos in Bulk SamplesSAI Global - APACNoch keine Bewertungen
- GMW14872 - Cyclic Corrosion Laboratory TestDokument22 SeitenGMW14872 - Cyclic Corrosion Laboratory TestZAPSENoch keine Bewertungen
- Clean ROOM HVACDokument40 SeitenClean ROOM HVACAjay Sastry0% (1)
- mp1 2 PDFDokument1 Seitemp1 2 PDFAmer MehmoodNoch keine Bewertungen
- Requisitions IndexDokument13 SeitenRequisitions IndexKarnan ThirugnanamNoch keine Bewertungen
- NGL Fractionation Using HYSYSDokument30 SeitenNGL Fractionation Using HYSYSAhmad Deyab100% (3)
- Catalogo Masel OrtodonciaDokument171 SeitenCatalogo Masel OrtodonciaJuan OntiverosNoch keine Bewertungen
- LaMotte 1756 Fluoride Tracer PockeTester InstructionsDokument16 SeitenLaMotte 1756 Fluoride Tracer PockeTester InstructionsPromagEnviro.comNoch keine Bewertungen
- AUtomotive Heat ExchangerDokument28 SeitenAUtomotive Heat ExchangersantoshkumarvenuNoch keine Bewertungen
- Aakash Rank Booster Test Series For NEET Aakash Rank Booster Test Series For NEET-2020 2020Dokument12 SeitenAakash Rank Booster Test Series For NEET Aakash Rank Booster Test Series For NEET-2020 2020VedNoch keine Bewertungen
- Model AG168 Standard Response Upright Sprinkler (SIN AG1124)Dokument4 SeitenModel AG168 Standard Response Upright Sprinkler (SIN AG1124)arieNoch keine Bewertungen
- EOCQ Ans 6Dokument2 SeitenEOCQ Ans 6harshanauoc100% (2)
- Columbia Lighting Product Selection Guide Edition 3 1996Dokument116 SeitenColumbia Lighting Product Selection Guide Edition 3 1996Alan MastersNoch keine Bewertungen
- Tea PDFDokument2 SeitenTea PDFLalit KalraNoch keine Bewertungen
- X RAY Residual StressDokument36 SeitenX RAY Residual StressAnonymous oTrMza100% (1)
- Clay Brick MakingDokument9 SeitenClay Brick MakingapihanasNoch keine Bewertungen
- Soil Freeze-Thaw Effects On Bank Erodibility and Stability: ElecteDokument23 SeitenSoil Freeze-Thaw Effects On Bank Erodibility and Stability: ElecteiliavaNoch keine Bewertungen
- Alcohols, Phenols and Ethers - MCQs Test - 3Dokument3 SeitenAlcohols, Phenols and Ethers - MCQs Test - 3Prasant KumarNoch keine Bewertungen
- Spider SilkDokument5 SeitenSpider SilkimranNoch keine Bewertungen
- NEET/JEE: 2020-21: Periodic PropertiesDokument3 SeitenNEET/JEE: 2020-21: Periodic Propertiesshantinath123gmailcoNoch keine Bewertungen