Beruflich Dokumente
Kultur Dokumente
Amyotrophic Lateral Sclerosis
Hochgeladen von
Aathi PathmanathanCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Amyotrophic Lateral Sclerosis
Hochgeladen von
Aathi PathmanathanCopyright:
Verfügbare Formate
Amyotrophic Lateral Sclerosis
Aathi Pathmanathan
PGY1
ALS
progressive neurodegenerative disorder that
causes muscle weakness, disability, and
eventually death, with a median survival of 3-
5years
Amyotrophy referring to the atrophy of
muscle fibers, which are denervated as their
corresponding anterior horn cells degenerate.
Lateral sclerosis refers to the changes seen
in the lateral columns of the spinal cord as
upper motor neuron (UMN) axons in these
areas degenerate and are replaced by fibrous
astrocytes (gliosis).
Epidemiology
Classified as either:
Sporadic ( 90-95% of cases)
Familial (5-10%)
Incidence and Prevalence
Incidence rates for ALS in N. America &
Europe range 1.5 -2.7 per 100,000/year,
Prevalence rates range between 2.7 -7.4 per
100,000
incidence lower among Asian, African and
Hispanics than Caucasians
Age: incidence increases with each decade,
esp. after age 40 years
peaks at age 74, decreasing after
incidence and mortality rates of ALS have
been slowly increasing over decades
?may be due to longer life expectancy
Risk factors
only established risk factors for ALS are age
and family history.
Some evidence suggests that cigarette
smoking is also a risk factor
weaker risk factors:
military service, agricultural work, factory work,
heavy manual labor, exposure to welding,
exposure to heavy metal, work in the plastics
industry, repetitive muscle use, athleticism,
playing professional soccer, trauma, electrical
shock, early-onset alopecia
?EtOH protective
Familial ALS
Familial ALS accounts for 5 to 10 percent of all
ALS cases
Most are autosomal dominant pattern of
inheritance.
Juvenile forms of ALS are more commonly
familial.
Mean age of onset is 10-20 years younger in
patients with familial ALS than in patients with
apparently sporadic disease
Age of onset may also be modified by genetic
factors independent of the cause of ALS
Gene Locus Protein Inheritance
SOD1 (ALS1) 21q22.11 Superoxide
dismutase 1 (SOD1)
AD*
ALS2 2q33 Alsin (ALS2) Juvenile/AR**
ALS3 18q21 Unknown AD
ALS4 9q34 SETX Juvenile/AD
ALSS 15q15 SPG11 Juvenile/AR
FUS (ALS6) 16p11.2 FUS AD
ALS7 20ptel-p13 Unknown AD
ALS8 20q13.3 VABP AD
ALS9 14q11.2 Angiogenin (ANG) AD
TARDBP (ALS10) 1p36.2 TAR DNA-binding
protein (TARDBP)
AD
ALS11 6q21 FIG4 AD
ALS12 10p13 OPTN AD; AR
ALS13 12q24 ATXN2 AD
ALSX Xp11 UBQLN2 X-linked
C9orf72 (ALS-FTD) 9q21-22 C9ORF72 AD
ALS-FTD 9p13.3 SIGMAR1 AD; Juvenile/AR
PFL1 17p13.2 Profilin 1 AD
*ADautosomal dominant; **ARautosomal recessive
PATHOLOGY
characterized by motor neuron degeneration
and death with gliosis replacing lost neurons.
Cortical motor cells (pyramidal and Betz cells)
disappear leading to retrograde axonal loss
and gliosis in the corticospinal tract.
This gliosis results in the bilateral white matter
changes sometimes seen in the brain
magnetic resonance imaging (MRI) of patients
with ALS.
The spinal cord becomes atrophic.
The ventral roots become thin, and there is a
loss of large myelinated fibers in motor
nerves. The affected muscles show
denervation atrophy with evidence of
reinnervation such as fiber type grouping.
Additional pathologic findings may include a
loss of frontal or temporal cortical neurons,
particularly in ALS with frontotemporal
dementia (ALS-FTD). Mounting evidence from
pathologic and genetic studies has led to the
hypothesis that similar molecular pathways
are involved in both disorders.
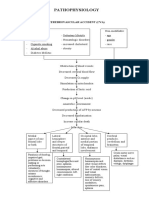
Pathophysiology
Etiology of amyotrophic lateral sclerosis (ALS) is
unknown.
A number of potential mechanisms have been
proposed including:
abnormal RNA processing
SOD1-mediated toxicity
excitotoxicity
cytoskeletal derangements
mitochondrial dysfunction
viral infections, apoptosis
growth factor abnormalities
inflammatory responses
SOD1-mediated toxicity
Superoxide dismutase type 1 (SOD1) is a
metalloenzyme that catalyzes the conversion
of toxic superoxide radicals to oxygen (O2)
and hydrogen peroxide (H2O2).
discovery that mutations in the SOD1 gene are
associated with some cases of familial ALS
suggested that free radical toxicity may play a
role in the process of neuronal cell death or
apoptosis
In addition, SOD1 mutations have been found
in 0.7 to 4 percent of patients with "sporadic"
ALS
Inflammatory responses
A number of studies have shown that
inflammatory processes are involved in ALS
disease progression and neuronal death
These inflammatory responses include
activation of microglia and astrocytes as well
as infiltration of natural killer cells, peripheral
T cells, and monocytes into the central
nervous system (CNS).
Many of these reports have focused on
microglia, which are immune-modulating cells
of the CNS. Once activated, microglia
elaborate a host of factors, including nitric
oxide, oxygen radicals, cytokines, glutamate,
and others that may play roles in part of the
cascade leading to motor neuron cell death
Several lines of evidence suggest that
microglial activation is triggered by mutant
SOD1 and contributes to neurodegeneration:
Microglial activation was found to be a prominent
part of the spinal cord pathology in several studies
employing transgenic mutant SOD1 mice
Mutant SOD1 appears to increase the production of
damaging reactive oxygen species by activated
microglia, thereby accelerating motor neuron injury
In spinal cord cultures, mutant SOD1 induced
activation of microglia, increasing their release of
proinflammatory cytokines and free radicals, leading
to secondary motor neuron injury
Misfolded mutant SOD1 activates caspase-1 and the
proinflammatory cytokine interleukin-1 in microglia
Excitotoxicity
excessive levels of the excitatory NTs glutamate may initiate a
cascade resulting in cell death of motor neurons in ALS.
Excessive activation of glutamate receptors may lead to
increased entry of calcium into cells.
In turn, intracellular Ca may trigger a cascade of events that causes neuronal
cell death via lipid peroxidation, nucleic acid damage, and mitochondrial
disruption.
In support of the excitotoxicity hypothesis is finding of
elevated glutamate levels in the CSF of pts with sporadic ALS
best evidence suggesting that glutamate excitotoxicity may
have a role in ALS disease progression is the demonstration
that the antiglutaminergic drug riluzole improves survival in
patients with the disease
Mitochondrial dysfunction
Mitochondrial dysfunction occurs early in the
transgenic mutant SOD1 mouse model,
preceding other evidence of motor neuron
damage
Data suggest that a selective recruitment of
mutant SOD1 to spinal cord mitochondria,
when compared with less affected tissues,
may explain the abundant pathology in the
spinal cord of the transgenic mutant SOD1
mouse
Human biochemical and morphologic studies
demonstrate consistent mitochondrial
abnormalities
These abnormalities may be the result of
oxidative stress and, in turn, may potentiate it.
Viral infections
Poliovirus, enteroviruses, and exogenous or
inherited endogenous retroviruses, have been
proposed to be causative agents of ALS
However, exhaustive studies have never
confirmed a clear etiologic relationship.
Human immunodeficiency virus (HIV) infection
and Lyme disease rarely cause an ALS-like
syndrome
Growth factors
Growth factors such as brain-derived
neurotrophic factor (BDNF), ciliary
neurotrophic factor (CNTF), and insulin-like
growth factor (IGF-1) have been studied
previously both for their possible role in the
etiology in ALS and for their therapeutic
potential
discovery that pts who have a specific
haplotype for vascular endothelial growth
factor (VEGF) may be more susceptible to ALS
shows possible role of GFs
Treatment of these animal models with VEGF
in a number of different paradigms has
resulted in significant improvement, thus
establishing VEGF as a potential candidate in
ALS pathogenesis and targeting it for use in
therapeutic studies
Clinical Features
combination of UMN and LMN
signs/symptoms
UMN findings of hyperreflexia and spasticity
result from degeneration of frontal motor
neurons located in the motor strip (Brodman
area 4) and their axons traversing the corona
radiata, internal capsule, cerebral peduncles,
pontine base, medullary pyramids, and the
lateral corticospinal tracts of the spinal cord
LMN findings of weakness, atrophy or
amyotrophy, and fasciculations are a direct
consequence of degeneration of lower motor
neurons in the brainstem and spinal cord
producing muscle denervation.
The loss of motor neurons results in the
primary clinical symptoms and signs of ALS.
These may produce impairment affecting limb,
bulbar, axial and respiratory function.
Differences in site and segment (cranial,
cervical, thoracic, or lumbosacral) of onset,
pattern and speed of spread, and the degree
of upper and/or lower motor neuron
dysfunction produce a disorder that is
remarkably variable between individuals
Initial Presentation
The initial clinical manifestation of ALS may
occur in any body segment (bulbar, cervical,
thoracic or lumbosacral)
Asymmetric limb weakness is the most
common presentation of ALS (80 %).
UE/LE frequency is 50/50
UE onset is most often begins with hand weakness
but may begin in the shoulder girdle muscles.
LE onset of ALS most often begins with weakness
of foot dorsiflexion (foot drop), while proximal
pelvic girdle onset is less common.
20% of pts have onset in the bulbar segment,
which most often presents with either
dysarthria or dysphagia.
Less common patterns of ALS onset include
respiratory muscle weakness (1 to 3 %),
generalized weakness in the limbs and bulbar
muscles (1 to 9 %), axial onset with head drop
or truncal extension weakness, and weight
loss with muscle atrophy, fasciculations, and
cramps
UMN symptoms
Loss of UMNs results in slowness of movement, incoordination and stiffness
UE UMN symptoms: poor dexterity with resulting difficulty performing activities of
daily living.
LE UMN symptoms: spastic gait with poor balance and may include spontaneous
leg flexor spasms and ankle clonus
Bulbar UMN symptoms:
UMN dysarthria characteristically strained vocal quality with slow speech.
UMN dysphagia results from slow and discoordinated contraction of the
swallowing muscles, which may lead to coughing and choking
pseudobulbar affect. inappropriate laughing, crying, or yawning.
Laryngospasm short-lived (usually <30 seconds) reflex closure of the larynx
that most often occurs in response to aspiration of food particles or liquids,
including saliva.
increased masseter tone
difficulty opening the mouth. When severe trismus.
Axial UMN dysfunction stiffness and imbalance
LMN symptoms
Loss of LMNs results in weakness, usually accompanied by atrophy and fasciculations
LMN loss in limbs:
Hand weakness causes difficulty manipulating small objects and using writing
instruments . Proximal arm weakness results in difficulty elevating the arm to the level
of the mouth or above the head.
Foot and ankle weakness results in tripping, foot drop slapping gait, and falling.
Proximal leg weakness results in difficulty arising from chairs, climbing stairs and
getting off of the floor. Balance may also be adversely affected.
Bulbar LMN signs: Dysarthria and dysphagia can also result from LMN damage
Dysarthria may result from weakness of the tongue, lips or palate.
Speech is usually slurred and may have a nasal quality.
Hoarseness due to associated vocal cord weakness.
Dysphagia results from tongue weakness with disruption of the oral phase of swallowing or
from pharyngeal constrictor weakness with disruption of the pharyngeal phase of
swallowing or both
Pharyngeal weakness: coughing and choking on food, liquids or secretions such as saliva or
mucus. Aspiration may result.
LMN symptoms
LMN weakness of the upper face may produce incomplete eye closure
In the lower face the result may be a poor lip seal that may contribute to drooling or
sialorrhea particularly in patients with associated swallowing difficulty. LMN weakness of the
masseter can cause difficulty chewing; when severe it may produce an inability to close the
mouth.
LMN weakness of the pterygoids may produce difficulty opening the mouth and moving the
jaw from side to side.
Severe masseter and pterygoid weakness may contribute to disarticulation of the
temporomandibular joint.
LMN weakness affecting the trunk and spine may produce difficulty holding up the head and
difficulty maintaining an erect posture
LMN weakness of the diaphragm produces progressive dyspnea with decreasing amounts of
effort culminating in dyspnea at rest and with talking along with reduced vocal volume
Diaphragmatic weakness may also result in orthopnea and sleep disordered breathing.
Cognitive symptoms
There is a well-established link between ALS
and frontotemporal executive dysfunction that
may precede or follow the onset of
upperand/or lower motor neuron dysfunction
The frequency of frontotemporal executive
dysfunction in patients with ALS varies in
previous reports from 28 -100 %.
Nevertheless, most patients with ALS do not
have overt dementia, and when present,
cognitive impairment is typically subtle
Retrospective data suggest that ALS with FTD
may be associated with shorter survival than
ALS with normal executive and behavioral
function
The presentation of FTD may be dramatic and
initially suggestive of a psychiatric disorder
because of change in personality, impairment
of judgment, and development of obsessional
behaviors.
Autonomic symptoms
Autonomic symptoms may occur in ALS as the
disease progresses
Constipation occurs frequently and is likely
multifactorial.
Delayed colonic motility has been demonstrated.
Dysphagia for thin liquids related to pharyngeal muscle
weakness may lead to dehydration that can exacerbate
constipation.
Symptoms of early satiety and bloating
consistent with delayed gastric emptying also
occur as the disease progresses
Urinary urgency without incontinence is
common, while incontinence is uncommon.
Some patients complain of excessive sweating
Parkinsonism and supranuclear
gaze palsy
EPS and signs of parkinsonism may precede
or follow the UMN/LMN symptoms.
EPS: may include facial masking, tremor,
bradykinesia, and postural instability.
At times, a supranuclear gaze abnormality
occurs that is similar to that seen in
progressive supranuclear palsy.
Preserved Functions
Certain motor neurons usually are spared in
ALS
Most patients retain EOM and bowel/bladder
control.
Pts may develop urge incontinence and
constipation because of weak abdominal
musculature, but sphincter control generally
unaffected.
Extraocular motor neurons residing in CN 3,4
and 6 nuclei are spared until very late in
disease.
Patients who choose long-term mechanical
ventilation have a longer clinical course that
can include progressive difficulty with ocular
motility.
Since the disease primarily involves motor
neurons, sensory function typically is
preserved, although a minority of patients
complains of some numbness and
paresthesias.
Abnormalities have been reported on sensory
NCS in a small number of patients with ALS,
but these findings often reflect the presence
of an unrelated, coexistent condition.
Clinical Patterns of Progression
ALS is a relentlessly progressive disorder with
a clinical course that is nearly always linear,
with a relatively constant slope.
While the rate of progression between
individuals is variable, the history should
reflect gradual and progressive worsening
over time without intervening remissions or
exacerbations.
Symptoms initially spread within the segment
of onset and then to other regions in a
relatively predictable pattern
In patients with unilateral arm onset the most
common (about 60 -70 %) pattern of spread is
to the contralateral arm, then to the ipsilateral
leg, then to the contralateral remaining leg,
and then to the bulbar muscles.
In patients with unilateral leg onset the most
common (about 60 to 70 %) pattern of spread
is to the contralateral leg, then to the
ipsilateral arm, then to the contralateral arm,
and then to bulbar muscles.
In patients with bulbar onset the most
common pattern of spread is to one arm and
then to the contralateral arm
Life threatening features
Progressive Neuromuscular Respiratory Failure
may be the first manifestation of the disease but more
commonly develops after months/years of progressive
limb and/or bulbar muscle weakness.
most common cause of death in ALS
Progressive Dysphagia :
may be one of the initial manifestations of the disease or may
develop after months/years of progressive limb and/or other
bulbar weakness.
poses a risk for aspiration of food, liquids, or secretions with
resultant pneumonia and may also lead to malnutrition and
dehydration. These conditions
can be minimized with PEG tube insertion.
SPECTRUM OF MOTOR NEURON
DISEASE
Amyotrophic lateral sclerosis is the most
common form of motor neuron disease and
includes both upper and lower motor neuron
pathology.
Progressive Muscular Atrophy
progressive LMN disorder
10% of MND
When the disease remains confined to the
lower motor neuron, survival may be
prolonged compared with classic ALS
median survival (48 versus 36 months)
Some individuals with PMA never develop
clinical upper motor neuron signs. Many,
however, develop upper motor neuron signs
later in their clinical course, at which point the
disease is called lower motor neuron-onset
ALS.
Typically, upper motor neuron involvement
occurred within two years of symptom onset.
At autopsy, patients with progressive muscular
atrophy who never developed clinically
apparent upper motor neuron signs frequently
have upper motor neuron pathology, including
corticospinal tract abnormalities
Primary Lateral Sclerosis
progressive isolated UMN disorder
Compared with ALS, it is characterized by
slower progression, lack of weight loss, and
absence of lower motor neuron findings on
examination or electromyography in the first
four years after symptom onset
Some individuals with primary lateral sclerosis
never develop clinical LMN signs. Most,
however, do develop LMN signs later in clinical
course UMN-onset ALS.
Pure primary lateral sclerosis and upper motor
neuron-dominant ALS appear to have a more
benign prognosis than typical ALS
Survival was longer and disease progression
slower in patients classified as primary lateral
sclerosis compared with ALS controls
Survival for patients with upper motor
neuron-dominant ALS was intermediate
between that of primary lateral sclerosis and
classic ALS
Progressive bulbar palsy
10% cases of MND
Progressive bulbar palsy is a progressive upper
and lower motor neuron disorder of cranial
muscles (CN 9-12)
may occasionally stay isolated to the bulbar
segment, but more commonly, upper and
lower motor neuron signs and symptoms
spread to involve other segments referred
to as bulbar-onset ALS.
Flail arm syndrome
flail arm syndrome (also called brachial
amyotrophic diplegia) is characterized by
progressive LMN weakness and wasting that
mainly affects the proximal arm
Proximal distal, to the point where arm and
hand function is severely impaired
often asymmetric
patients presenting with the flail arm variant
of ALS have a slower rate of progression both
to the spread of signs and symptoms in other
body segments and to development of
respiratory muscle weakness
Flail leg syndrome
Flail leg syndrome (also called the
pseudopolyneuritic variant
of ALS/motor neuron disease) is characterized
by progressive LMN weakness and wasting
with onset in distal leg
Patients presenting with the flail leg syndrome
have a slower rate of progression to
involvement of other body segments and of
the development of respiratory muscle
weakness
ALS-plus syndrome
Classically defined, ALS is considered a
degenerative disorder of the UMN/LMN, and
does not include symptoms/signs outside of
the voluntary motor system.
However, some patients have all of the clinical
features of ALS along with features of other
disorders:
frontotemporal dementia
autonomic insufficiency
parkinsonism
supranuclear gaze paresis
sensory loss.
Diagnosis
EMG
EMG findings in ALS combine features of acute
and chronic denervation and reinnervation
Acute denervation findings include
fibrillations and positive sharp waves.
Fasciculations in muscles with neurogenic
change are considered equivalent to
fibrillations and positive sharp waves.
Chronic denervation and reinnervation
findings include large amplitude, long
duration, complex motor unit action
potentials (MUAPs) with neurogenic
recruitment and a reduced interference
pattern. Instability of motor unit action
potentials indicative of recent reinnervation is
considered an important indication of ongoing
denervation and reinnervation
Fasciculation potentials may also appear in
denervated muscle and represent
spontaneous firing of motor units that are not
voluntarily recruited. Fasciculations may be
visible to the naked eye when they occur on
the surface of muscles
Nerve Conduction Studies
Sensory and motor nerve conduction studies
are most often normal in ALS, though
compound motor action potential (CMAP)
amplitudes may be reduced in severely
atrophic and denervated muscles
Motor unit number estimation is a nerve
conduction-based method that assesses the
number of viable motor axons innervating
small hand or foot muscles
Although this technique has applications to many
diseases, it has been applied most successfully to
ALS
Motor unit numbers drop prior to the onset of
clinical weakness, and changes in this measure
can be used as an outcome measure in clinical
trials
Neuroimaging
Neuroimaging has traditionally been used to
exclude other possible diagnoses in the
evaluation of suspected ALS.
MRI is the preferred modality, unless there is a
contraindication.
Brain MRI should be performed whenever
bulbar disease is present.
Cervical and lumbosacral spine MRI can be
used to evaluate lower motor neuron disease
in the arms and legs.
When evaluating upper motor neuron disease,
MRI should be performed in all segments
rostral to the clinical findings; this includes the
brain, cervical spine, and thoracic spine when
upper motor neuron findings are in the legs.
Conventional MRI is usually normal in ALS,
although increased signal in the corticospinal
tracts on T2-weighted and FLAIR images and
hypointensity of the motor cortex on T2-
weighted images has been reported
Lab Testing
Laboratory testing of blood, urine, and sometimes cerebrospinal fluid is performed routinely
during the evaluation of motor neuron disease.
Routine lab work usually includes complete blood count with differential, electrolytes including
calcium and phosphate, liver function tests, thyroid studies, creatine kinase, erythrocyte
sedimentation rate, antinuclear antibody, rheumatoid factor, vitamin B12, anti-GM1 antibody,
serum protein electrophoresis with immunofixation, and urine protein electrophoresis with
immunofixation.
In ALS, creatine kinase may be elevated up to approximately 1000 U/L on the basis of
denervation.
In patients with an elevated serum calcium level, the serum parathyroid hormone level should
be checked. ALS is rarely associated with primary hyperparathyroidism
Identification of a serum paraprotein should prompt further work-up with a 24-hour urine
protein electrophoresis, a skeletal survey, and CT of the chest, abdomen and pelvis to look for
myeloma and lymphoma.
Laboratory testing
Testing for HIV may be appropriate particularly in younger patients, at-risk individuals, and those
with atypical features.
Screening for heavy metals in the blood and urine is not required if there is no known
occupational exposure. Only lead intoxication has been reported to cause a condition resembling
lower motor neuron predominant ALS.
Testing for the antibodies found in myasthenia gravis (acetylcholine receptor antibodies and MuSK
antibodies) and Lambert-Eaton myasthenic syndrome (voltage-gated calcium channel antibodies) is
appropriate in the right clinical setting, and is particularly appropriate in patients with bulbar
dysfunction or any ocular motility disturbance.
Lumbar puncture and CSF analysis should be performed if there is clinical suspicion for Lyme
disease, HIV infection, or chronic inflammatory demyelinating polyneuropathy (CIDP).
Lumbar puncture for CSF analysis that includes cytology and a search for systemic malignancy
should be considered in lower motor neuron disorders with symptoms that have progressed over a
period of less than two years.
In addition, lymphoma and breast cancer can produce an indirect paraneoplastic degeneration of
the motor neurons that is most commonly subacute to chronic.
Muscle biopsy
Muscle biopsy is not a routine part of the
diagnostic evaluation of ALS but may be
performed if myopathy suspected on clinical,
electrodiagnostic, or serological grounds.
Treatment
Riluzole is the only drug to have any impact on
survival in ALS
2 CLINICAL TRIALS
1. In a prospective, double-blind, placebo-controlled
trial in 155 outpatients with ALS, survival at 12 months
was significantly higher for patients receiving
riluzole (100 mg/day) compared with controls (74
versus 58 % )
bulbar-onset ALS even greater adv. for survival at
12 months (73 versus 35 percent).
(Bensimon et al , 1994)
2. In a larger follow-up study, 959 patients with
clinically probable or definite ALS of less than 5 years
duration randomly assigned treatment with
riluzole (50 mg, 100 mg, or 200 mg daily) or placebo.
After median f/u of 18 months, the primary
outcome of survival significantly higher for riluzole-
treated group (100 mg/day) compared with controls
(57 versus 50 % , adjusted relative risk 0.65, 95% CI
0.50-0.85).
(Lacomblez, 1996)
3 separate mechanisms of riluzole thought to
reduce glutamate-induced excitotoxicity:
inhibition of glutamic acid release,
noncompetitive block of NMDA receptor
mediated responses, and direct action on the
voltage-dependent sodium channel
AAN Guidelines
Patients most likely to benefit from treatment
with riluzole include:
Definite or probable ALS by El-Escorial criteria, in
whom other causes of progressive muscle atrophy
have been ruled out
Symptoms present for < 5 years
Vital capacity (VC) greater than 60 percent of
predicted
No tracheostomy
Patients for whom no randomized data
support the use of riluzole but expert opinion
suggests potential benefit include those who
have
Suspected or possible ALS by El-Escorial criteria
Symptoms present > 5 years
VC less than 60 percent of predicted
Tracheostomy for prevention of aspiration only
(ventilator independent)
Expert consensus suggests riluzole is of
uncertain benefit in patients who have the
following conditions:
Tracheostomy required for ventilation
Other forms of anterior horn cell disease
Dosage and side effects
recommended dosage of riluzole is 50 mg BID
well absorbed orally with a bioavailability of
60 percent and an elimination half-life of 12
hours.
Metabolism is through the cytochrome P450
enzyme 1A2 (CYP1A2)
pharmacologic effects of riluzole may be
affected by inhibitors of CYP1A2, such
as theophylline and caffeine
ADVERSE EFFECTS:
Riluzole is well tolerated
Most common: asthenia, dizziness,
gastrointestinal disorders, and elevations in
liver enzyme activities
Neutropenia is extremely rare
Symptomatic Management of ALS
Spasticity Spastic muscle tone may be
useful to help patients maintain antigravity
power as muscle weakness progresses.
However, excess spasticity can be
uncomfortable and contribute to
incoordination of movement.
no high quality RCTs evaluating treatments
for spasticity in ALS/MND
studies suggest
that baclofen and tizanidine roughly
equivalent in efficacy and have similar rates of
adverse events.
Tizanidine is more often associated with dry
mouth, while baclofen is more often
associated with motor weakness.
suggested management options for spasticity
are:
Baclofen starting at 5 to 10 mg BID to TID;
doses up to 120 mg per day may be needed.
Tizanidine 2 to 4 mg by mouth BID up to a
total dose of 24 mg daily
RESPIRATORY MANAGEMENT
Pulmonary tests Patients with ALS should
have serial assessment of respiratory function
every three months starting at the time of
diagnosis.
A decrease of vital capacity (VC) to 50 percent
of predicted is often associated with
respiratory symptoms, and a VC of <25 to 30
percent of predicted is associated with
significant risk of respiratory failure or sudden
death.
Hypoxia and hypercarbia are late findings and
thus should not be relied upon as early
predictors of the need for mechanical
ventilation or other forms of respiratory
support
Noninvasive positive pressure
ventilation
Noninvasive positive pressure ventilation
(NPPV) is a therapeutic option for many
patients
especially useful if patients have symptoms of
respiratory compromise at night, but patients
often use it during waking hours as the
disease progresses.
Criteria for initiating noninvasive ventilation
include VC < 50 percent of predicted, or the
presence of orthopnea, or abnormal nocturnal
oximetry
Invasive ventilation
When long-term survival is the goal, invasive
ventilation requiring tracheostomy should be
offered.
Fewer than 10 percent of patients with ALS
choose invasive ventilation.
Invasive ventilation offered to:
Patients who present with respiratory failure and
who are otherwise largely neurologically intact
Patients in whom secretions cannot be managed,
and who therefore cannot benefit from
noninvasive ventilatory support
Patient preference
Muscle spasms
Quinine sulfate 325 mg BID was considered
the most effective treatment option for
muscle cramps concerns regarding severe
adverse events, including cardiac arrhythmias,
TCP , and severe hypersensitivity reactions
In lieu of quinine, suggested treatment
options for muscle spasms or cramps
include levetiracetam, carbamazepine and phe
nytoin.
Sialorrhea
Drooling is a common symptom in ALS caused
by the combination of facial muscle weakness
and reduced swallowing ability
(pseudohypersalivation).
Sialorrhea can be treated with the following :
Atropine 0.4 mg q4-6hrs
Hyoscyamine is available in several
formulations, including sustained release (0.375
to 0.75 mg every 12 hours; maximum 1.5 mg per
24 hours), fast acting oral (0.125 to 0.25 mg
every four hours or as needed before meals or
food; maximum 1.5 mg per 24 hours), and
transdermal (one or two patches every three
days)
Amitriptyline 10 to 150 mg once daily at bed
time
Glycopyrrolate 1 mg three times daily
Botulinum toxin injection into the salivary
glands appears to be safe and useful for treating
sialorrhea in patients with ALS
Low dose radiation therapy to the salivary
glands is suggested if sialorrhea does not
improve with pharmacologic therapy
DYSPHAGIA AND NUTRITION
Dysphagia increases risk for insufficient
caloric and fluid intake and worsening of
weakness and fatigue
It also increases the risk for aspiration and
choking.
Initial management of dysphagia by
modification of food and fluid consistency.
limited data suggest that percutaneous
gastrostomy tube (PEG) placement is
associated with prolonged survival, although
the degree of survival advantage is uncertain
For optimal safety and efficacy, PEG placed
before VC falls to 50 percent of predicted and
not in the preterminal phase, even if
dysphagia asymptomaticincreased
morbidity of the procedure as respiratory
function declines
Prognosis
Median survival is 3 years from clinical onset
of weakness.
15% of patients with ALS live 5 years after
diagnosis
5% survive > 10 years.
Long-term survival is associated with a
younger age at onset, male sex, and limb
(rather than bulbar) symptom onset.
Prognosis
Regionally limited forms of motor neuron
disease (ie, brachial biplegia, lumbosacral
biplegia, and progressive bulbar palsy [PBP]
that remains restricted) progress slower than
does classic ALS.
Progressive muscular atrophy (PMA), distinct
from classic ALS because of lack of upper
motor neuron (UMN) findings, progresses at
the same rate as classic ALS.
UMN-predominant ALS progresses at a slower
rate.
Survival in cases of primary lateral sclerosis
(PLS) is measured in decades.
These observations suggest that it is the loss
of LMNs that determines the prognosis.
Frontotemporal Dysfunction
Frontotemporal executive dysfunction may
precede or follow the onset of ALS, but most
patients with ALS do not have overt dementia,
and cognitive impairment is usually subtle
Approximately 15% of patients with ALS meet
criteria for frontotemporal dementia (FTD).
Patients with ALS associated with FTD have
shorter survival than do those with ALS alone
References
Bensimon G, Lacomblez L, Meininger V. A
controlled trial of riluzole in amyotrophic
lateral sclerosis. ALS/Riluzole Study Group. N
Engl J Med 1994; 330:585.
Lacomblez L, Bensimon G, Leigh PN, et al.
Dose-ranging study of riluzole in amyotrophic
lateral sclerosis. Amyotrophic Lateral
Sclerosis/Riluzole Study Group II. Lancet 1996;
347:1425.
Das könnte Ihnen auch gefallen
- Community Acquired Pneumonia, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsVon EverandCommunity Acquired Pneumonia, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsNoch keine Bewertungen
- Nasopharyngeal PathophysiologyDokument3 SeitenNasopharyngeal PathophysiologyMichael Anthony ReginaldoNoch keine Bewertungen
- SLE Risk Factors and ComplicationsDokument5 SeitenSLE Risk Factors and Complicationsjoyrena ochondraNoch keine Bewertungen
- PPPDokument3 SeitenPPPJack BangcoyoNoch keine Bewertungen
- Final - Mrs. X - Case Study (Group 3-RR21)Dokument45 SeitenFinal - Mrs. X - Case Study (Group 3-RR21)Gabriel Andrei C. RelayoNoch keine Bewertungen
- Lupus Case ReportDokument1 SeiteLupus Case ReportMendy HararyNoch keine Bewertungen
- History: Symptoms Associated With Specific Viral InfectionsDokument12 SeitenHistory: Symptoms Associated With Specific Viral InfectionsFatima Love Ariate-ArcasetasNoch keine Bewertungen
- CKD Case StudyDokument27 SeitenCKD Case StudyMary Rose Vito100% (1)
- Current Trends in The Mangement of Cerebrovascular Accident: Lecture NotesDokument58 SeitenCurrent Trends in The Mangement of Cerebrovascular Accident: Lecture NotesBoas WayneNoch keine Bewertungen
- CASE-STUDY-DM AmputationDokument32 SeitenCASE-STUDY-DM AmputationJerushamae Castillo0% (1)
- EncephalitisDokument30 SeitenEncephalitisHITIPHYSIO100% (1)
- Nursing Care PlanDokument6 SeitenNursing Care Planesteffie21Noch keine Bewertungen
- Understanding Stevens-Johnson Syndrome (SJSDokument53 SeitenUnderstanding Stevens-Johnson Syndrome (SJSKathrina CraveNoch keine Bewertungen
- Case Presentation - CKDDokument27 SeitenCase Presentation - CKDAmanda Padma50% (2)
- Rabies Case PresentationDokument36 SeitenRabies Case PresentationViorica Gavriliță100% (1)
- Personal Medical Records Document SummaryDokument16 SeitenPersonal Medical Records Document SummaryJayselle FelipeNoch keine Bewertungen
- CASE STUDY - Cerebral ContusionDokument20 SeitenCASE STUDY - Cerebral Contusionleyach80% (5)
- Case Study Cushing Syndrome 1Dokument5 SeitenCase Study Cushing Syndrome 1Selena Marie100% (1)
- Autoimmune EncephalitisDokument43 SeitenAutoimmune EncephalitisPrateek Kumar PandaNoch keine Bewertungen
- PathophysiologyDokument1 SeitePathophysiologynitlihpNoch keine Bewertungen
- Brain AbscessDokument5 SeitenBrain AbscessEugene Briagas Roque100% (1)
- Case Study - CVA With Bleeding (Medicine Ward)Dokument43 SeitenCase Study - CVA With Bleeding (Medicine Ward)Jonnah Lamson-Villarico100% (1)
- Encephalitis Causes, Symptoms & DiagnosisDokument55 SeitenEncephalitis Causes, Symptoms & DiagnosisAmit MartinNoch keine Bewertungen
- Case Presentation About Spinal Shock SyndromeDokument56 SeitenCase Presentation About Spinal Shock SyndromeAstral_edge010100% (1)
- Hiv Case StudyDokument2 SeitenHiv Case Studyapi-485814878Noch keine Bewertungen
- CVADokument116 SeitenCVAkathy100% (1)
- Colloid Nodular GoiterDokument37 SeitenColloid Nodular GoiterLori GeorgeNoch keine Bewertungen
- Case Study Systemic Lupus ErythematosusDokument54 SeitenCase Study Systemic Lupus ErythematosusCharmaine Paran100% (3)
- What Is Stroke?: BY: Luis Alberto Sanchez Hernandez Physical TherapistDokument12 SeitenWhat Is Stroke?: BY: Luis Alberto Sanchez Hernandez Physical TherapistLidiaAMonroyRNoch keine Bewertungen
- Head Injury GoulburnDokument47 SeitenHead Injury GoulburnSyarifah Maisyura100% (1)
- CVA Case StudyDokument9 SeitenCVA Case Studypylzanne100% (1)
- GRP 20 Final Abscess Case StudyDokument14 SeitenGRP 20 Final Abscess Case StudyBorja, Kimberly GraceNoch keine Bewertungen
- Pathognomonic Signs of Communicable Diseases: JJ8009 Health & NutritionDokument2 SeitenPathognomonic Signs of Communicable Diseases: JJ8009 Health & NutritionMauliza Resky NisaNoch keine Bewertungen
- CASE STUDY - CVA (Cerebrovascular Accident)Dokument28 SeitenCASE STUDY - CVA (Cerebrovascular Accident)'jmark FranciaNoch keine Bewertungen
- Case Study TbiDokument13 SeitenCase Study Tbiaica_184Noch keine Bewertungen
- Hydrocephalus UpdatesDokument65 SeitenHydrocephalus Updatescddinchimm100% (1)
- Primary AldosteronismDokument31 SeitenPrimary AldosteronismSteph100% (1)
- Hemorrhagic StrokeDokument18 SeitenHemorrhagic StrokeNdan RahmaNoch keine Bewertungen
- CardiomyopathyDokument45 SeitenCardiomyopathyHiLmy Zakiyah100% (1)
- Pulmonary EmbolismDokument16 SeitenPulmonary EmbolismniyigokNoch keine Bewertungen
- Group 8: Parik Rabasto Patel, D Patel, J Raghuwanshi Regis Moleta Moreno NaromalDokument35 SeitenGroup 8: Parik Rabasto Patel, D Patel, J Raghuwanshi Regis Moleta Moreno NaromalDominique RabastoNoch keine Bewertungen
- Rabies: Ragina AguilaDokument55 SeitenRabies: Ragina AguilaCharles Lester AdalimNoch keine Bewertungen
- Case Study Acute Lymphoid LeukemiaDokument1 SeiteCase Study Acute Lymphoid Leukemia2literNoch keine Bewertungen
- Pathophysiology of DMDokument4 SeitenPathophysiology of DMNicole Louise N. VillanuevaNoch keine Bewertungen
- Pathophysiology Acute Bacterial MeningitisDokument2 SeitenPathophysiology Acute Bacterial MeningitisNadira Farah PrayogoNoch keine Bewertungen
- MyelomeningoceleDokument7 SeitenMyelomeningocelemavefigNoch keine Bewertungen
- Anatomy and PhysiologyDokument10 SeitenAnatomy and PhysiologyChris CHris ChRis100% (1)
- Acute Myocardial InfarctionDokument20 SeitenAcute Myocardial InfarctionDavid Christian CalmaNoch keine Bewertungen
- Qtsoi Concept MapDokument5 SeitenQtsoi Concept MapGenella BabantoNoch keine Bewertungen
- Case PresentationDokument31 SeitenCase PresentationYogaPratayogaMNoch keine Bewertungen
- Case Study Cerebral InfarctDokument11 SeitenCase Study Cerebral InfarctRodel MorlaNoch keine Bewertungen
- Encephalitis PathophysiologyDokument19 SeitenEncephalitis PathophysiologyHeron Bayanin80% (5)
- Post-Cesarean and Bilateral Tubal Ligation Nursing CareDokument18 SeitenPost-Cesarean and Bilateral Tubal Ligation Nursing CaregenzpogiiNoch keine Bewertungen
- SHANS CFE 106b JOURNAL 1Dokument2 SeitenSHANS CFE 106b JOURNAL 1Shaima's VlogsNoch keine Bewertungen
- A Case Study Presentation On Systemic Lupus Erythematosus - Group 1Dokument34 SeitenA Case Study Presentation On Systemic Lupus Erythematosus - Group 1KeepItSecret100% (2)
- Guillain Barre SyndromeDokument16 SeitenGuillain Barre SyndromeAgnes NesiaNoch keine Bewertungen
- Bipolar Disorder Pathophysiology and GeneticsDokument11 SeitenBipolar Disorder Pathophysiology and GeneticsGirlly AlcantaraNoch keine Bewertungen
- Amyotrophic Lateral Sclerosis (ALS) : Kim Matias Noel Rabajante Rommuel Rebatis Sylene ReyesDokument22 SeitenAmyotrophic Lateral Sclerosis (ALS) : Kim Matias Noel Rabajante Rommuel Rebatis Sylene ReyesKimberly JaneNoch keine Bewertungen
- Neurodegener RalucaDokument88 SeitenNeurodegener RalucaAlexandra AnaellyNoch keine Bewertungen
- Current Concepts About Motr Neuron DiseaseDokument68 SeitenCurrent Concepts About Motr Neuron DiseaseMuhammad MuaazNoch keine Bewertungen
- 18 - Toronto Notes 2011 - NeurosurgeryDokument44 Seiten18 - Toronto Notes 2011 - NeurosurgeryErion Spaho100% (3)
- Vitamin DeficiencyDokument43 SeitenVitamin DeficiencyAathi Pathmanathan100% (1)
- Coping With Schizophreni A Guide For Patients Families and CaregiversDokument9 SeitenCoping With Schizophreni A Guide For Patients Families and CaregiversAathi PathmanathanNoch keine Bewertungen
- Antimicrobial ResistanceDokument6 SeitenAntimicrobial ResistanceAathi PathmanathanNoch keine Bewertungen
- StomasDokument2 SeitenStomasAathi PathmanathanNoch keine Bewertungen
- Dynamics and Neurocognitive Correlates of BPDDokument27 SeitenDynamics and Neurocognitive Correlates of BPDPriya PuriNoch keine Bewertungen
- Guidance Rapid Antibody COVID TestsDokument2 SeitenGuidance Rapid Antibody COVID TestsRaghu NadhNoch keine Bewertungen
- Oikawa's NPD and PTSDDokument2 SeitenOikawa's NPD and PTSDDannis NgoNoch keine Bewertungen
- Epilepsy GuidelinesDokument94 SeitenEpilepsy GuidelinesAyu Cliquers100% (1)
- Freelance Interactive Medical Advisor TestDokument2 SeitenFreelance Interactive Medical Advisor TestNiki Erista AyudiaNoch keine Bewertungen
- Guideline Karsinoma HepatoselulerDokument11 SeitenGuideline Karsinoma HepatoselulerMohammad Ihsan RifasantoNoch keine Bewertungen
- Bipolar Affective Disorder: Captain DR Yujal Man Singh Neuropsychiatrist, Shree Birendra HospitalDokument15 SeitenBipolar Affective Disorder: Captain DR Yujal Man Singh Neuropsychiatrist, Shree Birendra HospitalYujal Man SinghNoch keine Bewertungen
- The Complete Hematopathology GuideDokument113 SeitenThe Complete Hematopathology GuideJenny SNoch keine Bewertungen
- ModuleIV RespiratoryEmergencies CHF COPD AsthmaDokument96 SeitenModuleIV RespiratoryEmergencies CHF COPD AsthmaSaiKiranNoch keine Bewertungen
- Jerome Cauthen V3 PCDokument4 SeitenJerome Cauthen V3 PCSammy ChegeNoch keine Bewertungen
- Resume Ahmad AkbariDokument3 SeitenResume Ahmad AkbariSepideh MirzaeiNoch keine Bewertungen
- Appendicitis & AppendectomyDokument28 SeitenAppendicitis & Appendectomyhakunamatata_15100% (5)
- Tatalaksana Tekanan Tinggi Intrakranial Pada Anak-DikonversiDokument48 SeitenTatalaksana Tekanan Tinggi Intrakranial Pada Anak-DikonversiAbdurrahman Arsyad As SiddiqiNoch keine Bewertungen
- Animal in The BrainDokument10 SeitenAnimal in The BrainNathaly LapoNoch keine Bewertungen
- Pediatric TB Diagnosis UpdatesDokument39 SeitenPediatric TB Diagnosis UpdatesSiddhartha NandiNoch keine Bewertungen
- Pathology, Classification, and Grading of Neuroendocrine Neoplasms Arising in The Digestive System - UpToDateDokument45 SeitenPathology, Classification, and Grading of Neuroendocrine Neoplasms Arising in The Digestive System - UpToDatewipi112Noch keine Bewertungen
- Denture Stomatitis Causes and TreatmentDokument4 SeitenDenture Stomatitis Causes and TreatmentNovia SillaNoch keine Bewertungen
- EsophagusDokument41 SeitenEsophagusrayNoch keine Bewertungen
- L01 Obs. - History - Dr.a.rahim 2021 2022Dokument5 SeitenL01 Obs. - History - Dr.a.rahim 2021 2022salmaNoch keine Bewertungen
- Esophagostomy Tube Complications in Dogs and CatsDokument6 SeitenEsophagostomy Tube Complications in Dogs and CatsDwi MahdaNoch keine Bewertungen
- LoperamideDokument1 SeiteLoperamidekimglaidyl bontuyanNoch keine Bewertungen
- Oral Pathology in Paediatric Patients-Mini-Systematic ReviewDokument7 SeitenOral Pathology in Paediatric Patients-Mini-Systematic ReviewYuganya SriNoch keine Bewertungen
- Ayurvedic Diabetes CureDokument13 SeitenAyurvedic Diabetes CureYassine KrineNoch keine Bewertungen
- Palliative Care FormularyDokument12 SeitenPalliative Care FormularyRicardo FernandesNoch keine Bewertungen
- Difficulty Breathing, Cough, Discharge PlanDokument4 SeitenDifficulty Breathing, Cough, Discharge PlanSoleil Maxwell100% (1)
- PAVSDMAPCAsDokument3 SeitenPAVSDMAPCAsRajesh SharmaNoch keine Bewertungen
- SEHAT (Smart Health Tracker) : Inovasi Terbaru Untuk Memantau Kepatuhan Protokol Kesehatan Pada Anak Di Era Pandemi Covid-19 Ringkasan (Summary)Dokument3 SeitenSEHAT (Smart Health Tracker) : Inovasi Terbaru Untuk Memantau Kepatuhan Protokol Kesehatan Pada Anak Di Era Pandemi Covid-19 Ringkasan (Summary)Nurul IrhamnaNoch keine Bewertungen
- Rectal Exam Skill SheetDokument1 SeiteRectal Exam Skill SheetMuhammed ElgasimNoch keine Bewertungen
- Soft Tissue InjuryDokument72 SeitenSoft Tissue InjuryCucu Ne Eyang Kakung75% (8)
- Neonatal JaundiceDokument12 SeitenNeonatal JaundiceGiska T PutriNoch keine Bewertungen