Beruflich Dokumente
Kultur Dokumente
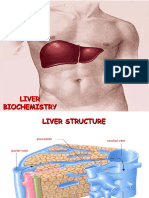
Biochemistry of Liver and Kidney
Hochgeladen von
AnastasiafynnCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Biochemistry of Liver and Kidney
Hochgeladen von
AnastasiafynnCopyright:
Verfügbare Formate
Biochemistry of
the liver and
kidney.
Urine formation.
Livers functions.
1. Liver is a main organ which is responsible for dividing of nutritional
substances in our organism (for example, glucose, triacylglicerides and
ketone bodies).
2. Hepatocytes synthesizes as lot of blood plasma proteins and lipoproteins,
low-weight bioactive substances (creatin, 25-oxicalciferol, hem), cholesterol.
3. Synthesis of urea (final product of nitrogen metabolism) also takes place in
the liver.
4. Liver synthesizes bile acids and excrete a bile into intestinal tract. This
process plays a very important role in lipids digestion and excretion of
cholesterin and some products of metabolism into intestine.
5. Liver play a big desintoxification role, inactivates endogenic and exogenic
substances (drugs, some hormones, different toxins).
6. Liver is a depo for iron, some another metals, vitamines A, D, E, B
12
, folic
acid.
Role of the liver in carbohydrate metabolism.
From intestine glucose pass into the liver, where
most part of it undergone the phosphorillation.
Glucose-6-phosphate formed in result of this
reaction, which catalyzed by two enzymes
hexokinase and glucokinase.
Glucose-6-phosphate is a key product of
carbohydrates metabolism. In the liver this
substance can metabolized into different ways
depend of livers and whole organisms
necessity.
1. Synthesis of glicogen. Content in the liver 70-100g
2. Glucose-6-phosphatase catalize dephosphorillation of glucose-6-
phosphate and free glucose formed
3. Excess of glucose-6-phosphate, which not used for synthesis of
glicogen and forming of free glucose
4. Glucose-6-phosphate decomposites for H
2
O and CO
2
, and free
energy for hepatocytes formed.
5. Part of glucose-6-phosphate oxidized in pentosophosphate cycle.
6. Hepatocytes content full set of gluconeogenesis necessary
enzymes. So, in liver glucose can be formed from lactate,
pyruvate, amino acids, glycerine. Gluconegenesis from lactate
takes place during intensive muscular work. Lactate formed from
glucose in muscles, transported to the liver, new glucose formed
and transported to the muscles
Role of the liver in lipid metabolism.
In the liver all processes of lipid metabolism take place. Most important of them
are following:
Lipogenesis (synthesis of fatty acids and lipids). Substrate for this process
acetyl-CoA, formed from glucose and amino acids, which are not used for
another purposes
Liver more active than another tissues synthesizes saturated and
monounsaturated fatty acids. Fatty acids then used for synthesis of lipids,
phospholipids, cholesterol ethers.
Liver play a central role in synthesis of cholesterin, because near 80 % of its
amount is synthesized there. Biosynthesis of cholesterin regulated by
negative feedback. When the level of cholesterin in the meal increases,
synthesis in liver decreases, and back to front. Besides synthesis regulated
by insulin and glucagon.
Liver is a place of ketone bodies synthesis. These substances formed from
fatty acids after their oxidation, and from liver transported to another tissues,
first of all to the heart, muscles, kidneys and brain
Role of the liver in protein metabolism.
Liver has full set of enzymes, which are necessary for
amino acids metabolism. Amino acids from food used in
the liver for following pathways:
1. Protein synthesis.
2. Decomposition for the final products.
3. Transformation to the carbohydrates and lipids.
4. Interaction between amino acids.
5. Transformation to the different substances with amino
group.
6. Release to the blood and transport to another organs
and tissues.
Liver syntesizes 100 % of albumines, 90 % of
1
-globulines, 75 % of
2
-globulines, 50 % of -
globulines, blood clotting factors, fibrinogen,
protein part of blood lipoproteins, such enzyme
as cholinesterase.
Liver can synthesize non-essential amino acids.
Liver synthesizes purine and pyrimidine
nucleotides, hem, creatin, nicotinic acid, cholin,
carnitin, polyamines.
Role of the liver in detoxification processes.
A xenobiotics is a compound that is foreign to the body. The principal
classes of xenobiotics of medical relevance are drugs, chemical
cancerogens, and various compounds that have found their way into
our environment by one route or another (insecticides, herbicides,
pesticides, food additions, cosmetics, domestic chemical
substances).
Some internal substances also have toxic properties (for example,
bilirubin, free ammonia, bioactive amines, products of amino acids
decay in the intestine).
Moreover, all hormones and mediatores must be inactivated.
Reactions of detoxification take place in the liver.
Big molecules like bilirubin excreted with the bile to intestine and
leaded out with feces. Small molecules go to the blood and excreted
via kidney with urine.
The metabolism of xenobiotics has 2 phases:
In phase 1, the major reaction involved is
hydroxylation, catalyzed by members of a class
of enzymes referred to as monooxygenases or
cytochrome P-450 species. These enzymes can
also catalyze deamination, dehalogenation,
desulfuration, epoxidation, peroxidation and
reduction reaction. Hydrolysis reactions and
non-P-450-catalyzed reactions also occur in
phase 2.
In phase 2, the hydroxylated or other compounds produced in phase 1
are converted by specific enzymes to various polar metabolites by
conjugation with glucuronic acid, sulfate, acetate, glutathione, or
certain amino acids, or by methylation.
In certain cases, phase 1 metabolic reaction convert xenobiotics from
inactive to biologically active compounds. In these instances, the
original xenobiotics are referred to as prodrugs or procarcinogens. In
other cases, additional phase 1 reactions convert the active
compounds to less active or inactive forms prior to conjugation. In
yet other cases, it is the conjugation reactions themselves that
convert the active product of phase 1 to less active or inactive
species, which are subsequently excreted in the urine or bile. In a
very few cases, conjugation may actually increase the biologic
activity of a xenobiotics.
There are at least 5 types of phase 2 reactions:
1. Glucuronidation. UDP-glucuronic acid is the
glucuronyl donor, and a variety of glucuronyl
transferases, present in both the ER and cytosol, are
the catalysts. Molecules such as bilirubin, thyroxin, 2-
acetylaminofluorene (a carcinogen), aniline, benzoic
acid, meprobromate (a tranquilizer), phenol, crezol,
indol and skatol, and many steroids are excreted as
glucuronides. The glucuronide may be attached to
oxygen, nitrogen, or sulfur groups of substrates.
2. Sulfation. Some alcohols, arylamines, and phenols are
sulfated. The sulfate donor in these and other biologic
sulfation reactions is adenosine 3-phosphate-5-
phosphosulfate (PAPS); this compound is called active
sulfate
3. Conjugation with Glutathione. Glutathione (-
glutamylcysteinylglycine) is a tripeptide consisting of
glutamic acid, cysteine, and glycine. Glutathione is
commonly abbreviated to GSH; the SH indicates the
sulfhydryl group of its cysteine and is the business part
of the molecule. A number of potentially toxic
electrophilic xenobiotics (such as certain carcinogens)
are conjugated to the nucleophilic GSH. The enzymes
catalyzing these reactions are called glutathione S-
transferases and are present in high amounts in liver
cytosol and in lower amounts in other tissues.
Acetylation. These reactions is represented by X + Acetyl-CoA Acetyl-X +
CoA, where X represents a xenobiotic. These reactions are catalyzed by
acetyltransferases present in the cytosol of various tissues, particularly liver.
The different aromatic amines, aromatic amino acids, such drug as
isoniazid, used in the treatment of tuberculosis, and sulfanylamides are
subjects to acetylation. Polymorphic types of acetyltransferases exist,
resulting in individuals who are classified as slow or fast acetylators, and
influence the rate of clearance of drugs such as isoniazid from blood.
5. Methylation. A few xenobiotics (amines, phenol, tio-substances, inorganic
compounds of sulphur, selen, mercury, arsenic) are subject to methylation
by methyltransferases, employing S-adenosylmethionine as methyl donor.
Also catecholamines and nicotinic acid amid (active form of vitamin PP) are
inactivated due to methylation.
Very important way of detoxification is ureogenes (urea synthesis). Free
ammonia, which formed due to metabolism of amino acids, amides and
amines, removed from organism in shape of urea.
Kidney the couple organ, which is responsible for excriting of final
products of metabolism and for homeostasis. They regulate water
and mineral metabolism, acid-base balance, excriting of nitrogenous
slags, osmotic pressure. Also they regulate arterial pressure and
erhythropoesis.
Nephron is the structural and functional unit of kidney.
Urine fluid with different organic and inorganic compouds, which
must be excreted (excess of water, final products of nitrogen
metabolism, xenobiotics, products of proteins decay, hormones,
vitamins and their derivates). Most of them present in urine in a
bigger amount than in blood plasma. So, urine formation is not
passive process (filtration and diffusion only).
In basis of urine formation lay 3 processes: filtration, reabsorbtion and
secretion.
Glomerulal filtration. Water and low weight molecules go to the urine
with help of following powers: blood hydrostatic pressure in
glomerulas (near 70 mm Hg), oncotic pressure of blood plasma
proteins (near 30 mm Hg) and hydrostatic pressure of plasma
ultrafiltrate in glomerulal capsule (near 20 mm Hg). In normal
conditions, as You see, effective filtration pressure is about 20 mm
Hg.
Primary urine formed in result of filtration (about 200 L per day).
Between all blood plasma substances only proteins dont present in
a primary urine. Most of these substances are undergone to the
following reabsorbtion. Only urea, uric acid, creatinin, and other final
products of different metabolic pathways arent undergone to the
reabsorbt
For evaluate of filtration used clearance (clearance for some
substance it is a amount of blood plasma in ml, which is cleaned
from this substance after 1 minute passing through kidney).
Reabsorbtion. Lenght of renal tubules is about 100 km. So, all important for our organism are
reabsorbed during passing these tubules. Epitelium of renal tubules reabsorb per day 179 L of
water, 1 kg of NaCl, 500 g of NaHCO
3
, 250 g of glucose, 100 g of free amino acids.
All substances can be divided into 3 group:
1. Actively reabsorbed substances.
2. Substances, which are reabsorbed in a little amount.
3. Non-reabsorbed substances.
To the first group belong Na
+
, Cl
-
, Mg
2+
, Ca
2+
, H
2
O, glucose and other monosaccharides, amino
acids, inorganic phosphates, hydrocarbonates, low-weight proteins, etc.
Na
+
reabsorbed by active transport to the epitelium cell, then into the extracellular matrix. Cl
-
and HCO
3
-
following Na
+
according to the electroneutrality principle, water according to the
osmotic gradient. From extracellular matrix substances go to the blood vessels. Mg
2+
and Ca
2+
are
reabsorbed with help of special transport ATPases. Glucose and amino acids use the energy of
Na+ gradient and special carriers. Proteins are reabsorbed by endocytosis.
Urea and uric acid are little reabsorbable substances.
Creatinin, mannitol, inulin and some other substances are non-reabsorbable.
Some substances (K
+
, ammonia and other) are secreted into urine in the distal part of tubules. K
+
is
changed to Na
+
by the activity of Na
+
-K
+
ATPase.
Peculiarities of biochemical processes in kidney.
Kidney have a very high level of metabolic processes. They
use about 10 % of all O
2
, which used in organism.
During 24 hours through kidney pass 700-900 L of blood.
The main fuel for kidney are carbohydrates. Glycolysis,
ketolysis, aerobic oxidation and phophorillation are very
intensive in kidney. A lot of ATP formed in result.
Kidney have plenty of different enzymes: LDG (1, 2, 3, 5),
AsAT, AlAT. Specific for kidney is alanine amino
peptidase, 3rd isoform.
Regulation of urine formation.
Na-uretic hormone (produced in heart) decrease
reabsorbtion of Na
+
, and quantity of urine increased.
Aldosteron and some other hormones (vasopressin, renin,
angiotensin II) increase Na-reabsorbtion and decrease
quantity of urine.
Role of kidney in acid-base balance regulation.
Kidney have some mechanisms for maintaining acid-base
balance. Na
+
reabsorbtion and H
+
secretion play very
important role.
1. Primary urine has a lot of Na
2
HPO
4
(in dissociated form).
When Na
+
reabsorbed, H
+
secreted into urine and
NaH
2
PO
4
formed.
2. Formation of hydrocarbonates. Inside renal cells
carboanhydrase forms from CO
2
and H
2
O
H
2
CO
3
, which dissociated to H
+
and HCO
3
-
. H
+
excreted from cell into urine (antiport with Na
+
)
and leaded with urine. Na
+
connect with HCO
3
-
,
NaHCO
3
formed and go to the blood, thereupon
acidity decreased.
3. Formation of free ammonia. NH
3
used for
formation of NH
4
+
(H
+
ion associted), and
different acid metabolites excreted as ammonia
salts.
Role of kidney in water balance regulation.
Excessive entrance of water leads to dilution of
extracellular fluid. Decreasing of osmolality inhibits
secretion of antidiuretic hormone. Walls of collective
tubules stay non-penetrated to water and dilutive urine
formed.
If volume of blood circulation increases, circulation in
kidney increases also and hyperosmotic medium of
kidney medulla removed. Some substances in these
conditions return into blood. So, excess of water carried
with urine and a lot of soluble substances are
reabsorbed into blood. After water loading stopped,
hyperosmolality in kidney medulla returns for previous
stage during some days.
Physical and chemical characteristics of urine.
Urine amount (diures) in healthy people is 1000-2000 ml per day. Day-time
diures is in 3-4 times more than night-time.
Normal colour of urine is yellow (like hay or amber), what is due to presence of
urochrom (derivate of urobilin or urobilinogen). Some another colour
substances are uroerythrin (derivate of melanine), uroporphyrines,
rybophlavine and other. Colour depends from urine concentration.
Urine is transparent. This characteristic depends from amount of different salts
(oxalates, urates, phosphates), amount of present epitelium cells and
leucocytes.
Density of urine depends from concentration of soluble substances. Borders of
variation are from 1002 to 1035 g/l. Near 60-65 g of hard substances are
excreted with urine per day.
In normal conditions urine has acid or weak acid reaction (pH=5,3-6,8). This
depends from presence of NaH
2
PO
4
and KH
2
PO
4
.
Fresh urine has a specific smell, which is due to presence of flying acids. But a
lot of microorganisms, which are present in urine, split urea and free
ammonia formed.
Organic compounds of urine.
Proteins. Healthy people excretes 30 mg of proteins per day. As a rule these are low
weight proteins.
Urea. This is main part of organic compounds in urine. Urea nitrogen is about 80-90 % of
all urine nitrogen. 20-35 g of urea is excreted per day in normal conditions.
Uric acid. Approximately 0,6-1,0 g of uric acid is excreted per day in form of different
salts (urates), mainly in form of sodium salt. Its amount depends from food.
Creatinin and creatin. Near 1-2 g of creatinin is excreted per day, what depended from
weight of muscles. This is the constant for each person. Men excrete 18-32 mg of
creatinin per 1 kg of body weight per day, women 10-25 mg. Creatinin is non-
reabsorbable substance, so this test used for evaluating of renal filtration.
Amino acids. Per day healthy person excretes 2-3 g of amino acids (free amino acids
and different low weight molecule peptides). Also products of amino acids metabolism
can be found in the urine.
Couple substances. Hypuric acid (benzoyl glycine) is excreted in amount 0,6-1,5 g per
day. This index increases after eating a lot of berries and fruits, and in case of
proteins decay in the intestines.
Indican (potassium salt of indoxylsulfuric acid). Per day excrition of indican is about 10-25 g. Increasing of indicans
level in urine is due to inrtensification of decay proteins in the intestines and chronic diseases, which are
accompanied by intensive decopmosition of proteins (tuberculosis, for example).
Organic acids. Formic, acetic, butyric, -oxybutyric, acetoacetic and some other organic acids are present in urine in a
little amount.
Vitamines. Almost all vitamines can be excreted via kidney, especially, water-soluble. Approximately 20-30 mg of vit C,
0.1-0.3 mg of vit B1, 0.5-0.8 mg of vit B2 and some products of vitamines metabolism. These data can be used
for evaluating of supplying our organism by vitamines.
Hormones. Hormones and their derivates are always present in urine. Their amount depends from functional state of
endocrinal glands and liver. There is a very wide used test determination of 17-ketosteroids in urine. For healthy
man this index is 15-25 g per day.
Urobilin. Present in a little amount, gives to urine yellow colour.
Bilirubin. In normal conditions present in so little amount that cannot be found by routine methods of investigations.
Glucose. In normal conditions present in so little amount that cannot be found by routine methods of investigations.
Galactose. Present in the newborns urine, when digestion of milk or transformation of glalactose into glucose in the
liver are violated.
Fructose. It is present in urine very seldom, after eating a lot of fruits, berries and honey. In all other cases it indicates
about livers disorders, diabetes mellitus.
Pentoses. Pentoses are excreted after eating a lot of fruits, fruit juices, in case of diabetes mellitus and steroid
diabetes, some intoxications.
Ketone bodies. In normal conditions urine contains 20-50 mg of ketone bodies and this amount cannot be found by
routine methods of clinical investigations.
Porphyrines. Urine of healthy people contains a few I type porphyrines (up to 300 mkg per day).
Inorganic compounds of urine.
Urine of healthy people contains 15-25 g of inorganic compounds.
NaCl. Per day near 8-16 g of NaCl excreted with urine. This amount depends from amount of NaCl in
food.
Potassium. Twenty-four hours urine contains 2-5 g of K, which depends of amount of plants in the
food.
Different drugs can change excretion of Na and K. For example, salicylates and cortikosteroids keep
Na and amplify excretion of K.
Calcium. Twenty-four hours urine contains 0.1-0.3 g, which depends from content of calcium in the
blood.
Magnesium. Content of magnesium in urine is 0.03-0.18 g. So little amount of calcium and
magnesium in urine can be explained by bad water solubility of their salts.
Iron. Amount of iron in urine is about 1 mg per day.
Phosphorus. In urine are present one-substituted phosphates of potassium and sodium. Their
amount depends from blood pH. In case of acidosis two-substituted phosphates (Na
2
HPO
4
) react
with H
+
and one-substituted phosphates (NaH
2
PO
4
) formed. In case of alkalosis one-substituted
phosphates react with bases and two-substituted formed. So, in both cases amount of phosphates
in urine increases.
Sulfur. Amount of sulfur in twenty-four hours urine is 2-3 g per day (in form of SO
4
2-
).
Ammonia. Ammonia is excreted in ammonium sulfates and couple substances. Ammonium salts
make up 3-6 % of all nitrogen in urine. Their amount depends from character of food and blood
pH.
Das könnte Ihnen auch gefallen
- Lecture 12. Liver Biochemistry. Pigmentary Metabolism. JaundicesDokument43 SeitenLecture 12. Liver Biochemistry. Pigmentary Metabolism. JaundicesВіталій Михайлович НечипорукNoch keine Bewertungen
- 1 - Biochemistry Notes by Dr. M A Bari Siddiqui: DrmentorsDokument3 Seiten1 - Biochemistry Notes by Dr. M A Bari Siddiqui: DrmentorsShiva KumarNoch keine Bewertungen
- Biochemical Functions of LiverDokument82 SeitenBiochemical Functions of Liversiwap34656Noch keine Bewertungen
- Biotransfermation of XenobioticsDokument34 SeitenBiotransfermation of XenobioticsМохіт Кумар ЯмпатіNoch keine Bewertungen
- Biochemical Functions of LiverDokument94 SeitenBiochemical Functions of LiverMi PatelNoch keine Bewertungen
- Biotransformation and Excretion of DrugDokument63 SeitenBiotransformation and Excretion of DrugIzzuddin AhmadNoch keine Bewertungen
- Metabolic Functions of The LiverDokument3 SeitenMetabolic Functions of The LiverAizat KamalNoch keine Bewertungen
- Lecture 2 Drug Metabolism Phase IIDokument27 SeitenLecture 2 Drug Metabolism Phase IIfeegame7Noch keine Bewertungen
- Tissue MetabolismDokument13 SeitenTissue MetabolismTonny MutisyaNoch keine Bewertungen
- Amino Acids Metabol Synth of UreaDokument32 SeitenAmino Acids Metabol Synth of UreaAnastasiafynn100% (1)
- Metabolism 2017Dokument96 SeitenMetabolism 2017aimaNoch keine Bewertungen
- CHAPTER 5 MetabolismeDokument46 SeitenCHAPTER 5 MetabolismeYuli Irvaransiah DIatun NIkmahNoch keine Bewertungen
- Biochemistry of Specialized Tissues (Liver)Dokument24 SeitenBiochemistry of Specialized Tissues (Liver)Uloko ChristopherNoch keine Bewertungen
- Types of Reactions of Biotransformations of Xenobiotics in A Liver?Dokument3 SeitenTypes of Reactions of Biotransformations of Xenobiotics in A Liver?jimmyNoch keine Bewertungen
- Drug MetabolismDokument46 SeitenDrug Metabolismأحمد عاطفNoch keine Bewertungen
- BiochemieDokument10 SeitenBiochemiearian.boostani2002Noch keine Bewertungen
- Intergrated MetabolismDokument117 SeitenIntergrated MetabolismDerick SemNoch keine Bewertungen
- Biochemistry: Proteins and Amino AcidsDokument8 SeitenBiochemistry: Proteins and Amino AcidsrajanOrajanNoch keine Bewertungen
- Working Problem 5 - Semester 1, 2016: Medical ScienceDokument8 SeitenWorking Problem 5 - Semester 1, 2016: Medical Sciencedannyboy_588Noch keine Bewertungen
- Drug EliminationDokument18 SeitenDrug EliminationBriana NdayisabaNoch keine Bewertungen
- UntitledDokument14 SeitenUntitledSyafrianda PanggabeanNoch keine Bewertungen
- Unit 8 Digestion and AbsorptionDokument33 SeitenUnit 8 Digestion and AbsorptionGlory MuedaNoch keine Bewertungen
- CarbohydratesDokument19 SeitenCarbohydratesNaina YadavvNoch keine Bewertungen
- Chapter 44 LiverDokument17 SeitenChapter 44 LiverLiteriaNoch keine Bewertungen
- BCH 220 - DR ErhunseDokument6 SeitenBCH 220 - DR ErhunseSuccess OlamideNoch keine Bewertungen
- Lipids Digestion and AbsorptionDokument28 SeitenLipids Digestion and AbsorptionEng Matti Ur RehmanNoch keine Bewertungen
- METABOLISM OF XENOBIOTICS-revDokument28 SeitenMETABOLISM OF XENOBIOTICS-revEdo Pramana PutraNoch keine Bewertungen
- Biochemistry - Protein and LipidsDokument31 SeitenBiochemistry - Protein and LipidsGiorgi TamazashviliNoch keine Bewertungen
- Pharmacology: Modern Pharmacology With Clinical Application (6th Ed., P. 34) - IllinoisDokument7 SeitenPharmacology: Modern Pharmacology With Clinical Application (6th Ed., P. 34) - IllinoisTazkiyatan IsriaNoch keine Bewertungen
- Approval SheetDokument24 SeitenApproval SheetIntan AnggrainiNoch keine Bewertungen
- 1 - XenobioticsDokument24 Seiten1 - XenobioticsgeenaksamuelNoch keine Bewertungen
- Biochemistry - Fundamentals of Energy Exchange MSCDokument33 SeitenBiochemistry - Fundamentals of Energy Exchange MSCmariam1.ashrafNoch keine Bewertungen
- METABLSMDokument6 SeitenMETABLSMapi-3807124Noch keine Bewertungen
- Briefly Summarize The Involving Steps in The ATP SynthesisDokument7 SeitenBriefly Summarize The Involving Steps in The ATP SynthesisXuân ViNoch keine Bewertungen
- Biochemistry Review Booklet 1Dokument28 SeitenBiochemistry Review Booklet 1vaegmundigNoch keine Bewertungen
- Organic Pharmaceutical Chemistry I: by Dr. Mazin Albasrawy Lec. 1Dokument25 SeitenOrganic Pharmaceutical Chemistry I: by Dr. Mazin Albasrawy Lec. 1Mohammed Ahmed ShNoch keine Bewertungen
- 4) Conversion of An Active Compound To Another Equally Active Compound (No Change in Activity)Dokument5 Seiten4) Conversion of An Active Compound To Another Equally Active Compound (No Change in Activity)Surjeet ChauhanNoch keine Bewertungen
- Introduction To Anabolic Metabolism Lipid MetabolismDokument21 SeitenIntroduction To Anabolic Metabolism Lipid MetabolismAnna Sofia ReyesNoch keine Bewertungen
- Metabolism Complete Notes #KigogoDokument145 SeitenMetabolism Complete Notes #KigogoHesbone AneneNoch keine Bewertungen
- Biochem 2.1 Introduction To MetabolismDokument5 SeitenBiochem 2.1 Introduction To Metabolismlovelots1234Noch keine Bewertungen
- Cholesterol SphingolipidsDokument31 SeitenCholesterol SphingolipidsMaryam KNoch keine Bewertungen
- Carbohydrate MetabolismDokument4 SeitenCarbohydrate MetabolismMuhammad Naveed SheikhNoch keine Bewertungen
- Biochemistry of Digestive SystemDokument17 SeitenBiochemistry of Digestive SystemnishibuchiNoch keine Bewertungen
- Sugar MetabolismDokument113 SeitenSugar MetabolismmismaelNoch keine Bewertungen
- Drug Metabolism-LectDokument35 SeitenDrug Metabolism-LectFiona OyatsiNoch keine Bewertungen
- Elimination of DrugsDokument39 SeitenElimination of Drugsdeepshah6068Noch keine Bewertungen
- Other Foreign Substances These Substances Have Entered The Blood Supply EitherDokument15 SeitenOther Foreign Substances These Substances Have Entered The Blood Supply EitherAnis Assila Mohd RohaimiNoch keine Bewertungen
- t1 and UsherDokument8 Seitent1 and UsherTk MakotoseNoch keine Bewertungen
- Anabolism - Microbial MetabolismDokument28 SeitenAnabolism - Microbial MetabolismDni AwatifNoch keine Bewertungen
- 3rd Module 1Dokument44 Seiten3rd Module 1KARTHIK VINODNoch keine Bewertungen
- Biochem Q.B III Sess Mar 2020Dokument39 SeitenBiochem Q.B III Sess Mar 2020Jinal Jyoti JoshiNoch keine Bewertungen
- Protein - Metabolism PDFDokument25 SeitenProtein - Metabolism PDFMeika Kuruhashi67% (3)
- Metabolism of Essential AADokument7 SeitenMetabolism of Essential AA48 Syeda KainatNoch keine Bewertungen
- 1st Principle of GastroenterologyDokument191 Seiten1st Principle of GastroenterologyneysaonlineNoch keine Bewertungen
- Metabolic ProcessesDokument6 SeitenMetabolic ProcessesMark Andrian PontanalNoch keine Bewertungen
- Biological MoleculesDokument6 SeitenBiological MoleculessanaullahNoch keine Bewertungen
- Biochemistry of Kidneys and UrineDokument18 SeitenBiochemistry of Kidneys and UrineAndrias PutriNoch keine Bewertungen
- Biotransformation TYDokument61 SeitenBiotransformation TYabutalibNoch keine Bewertungen
- Turnitin Originality ReportDokument9 SeitenTurnitin Originality ReportfynneroNoch keine Bewertungen
- VisualDokument17 SeitenVisualAnastasiafynnNoch keine Bewertungen
- It SpecialistDokument3 SeitenIt SpecialistAnastasiafynnNoch keine Bewertungen
- My CVDokument4 SeitenMy CVAnastasiafynnNoch keine Bewertungen
- Schools JobsDokument3 SeitenSchools JobsAnastasiafynnNoch keine Bewertungen
- Anaesthesia Resident's HandbookDokument445 SeitenAnaesthesia Resident's HandbookAnastasiafynn50% (2)
- CirculationDokument4 SeitenCirculationAnastasiafynnNoch keine Bewertungen
- CCS - Handbook of Anesthesiology (2005)Dokument180 SeitenCCS - Handbook of Anesthesiology (2005)Rojelle LezamaNoch keine Bewertungen
- Us 134Dokument2 SeitenUs 134452bobNoch keine Bewertungen
- Sym DromeDokument4 SeitenSym DromeAnastasiafynnNoch keine Bewertungen
- YawsDokument15 SeitenYawsAnastasiafynnNoch keine Bewertungen
- Behaviourial ScienceDokument4 SeitenBehaviourial ScienceAnastasiafynnNoch keine Bewertungen
- HirsutismDokument22 SeitenHirsutismAnastasiafynnNoch keine Bewertungen
- Type 1 Diabetes: Endocrine Glands Insulin Pump Glucose Test Insulin Pump Type I DiabetesDokument9 SeitenType 1 Diabetes: Endocrine Glands Insulin Pump Glucose Test Insulin Pump Type I DiabetesAnastasiafynnNoch keine Bewertungen
- Biochemistry of Bone Tissueдокумент Microsoft WordDokument19 SeitenBiochemistry of Bone Tissueдокумент Microsoft WordAnastasiafynnNoch keine Bewertungen
- CarbohydratesDokument16 SeitenCarbohydratesAnastasiafynnNoch keine Bewertungen
- CellMembrane Structure and Functional PropertiesDokument5 SeitenCellMembrane Structure and Functional PropertiesAnastasiafynnNoch keine Bewertungen
- Biochemistry of Liver and KidneyDokument24 SeitenBiochemistry of Liver and KidneyAnastasiafynnNoch keine Bewertungen
- Blood Circulation System and It Regulation. Blood Circulation in Facial RegionDokument23 SeitenBlood Circulation System and It Regulation. Blood Circulation in Facial RegionAnastasiafynnNoch keine Bewertungen
- Biochemistry of The Liver and Kidney. Urine Formation.Dokument22 SeitenBiochemistry of The Liver and Kidney. Urine Formation.AnastasiafynnNoch keine Bewertungen
- Biochemistry of Blood, Muscle and Connective Tissue.Dokument21 SeitenBiochemistry of Blood, Muscle and Connective Tissue.Anastasiafynn100% (1)
- Biochemistry - As Science - Biomolecules. Structure and Properties of Enzymes - Isoenzymes.Dokument41 SeitenBiochemistry - As Science - Biomolecules. Structure and Properties of Enzymes - Isoenzymes.AnastasiafynnNoch keine Bewertungen
- Water and Sodium BalanceDokument4 SeitenWater and Sodium Balanceapi-3712326Noch keine Bewertungen
- Blood Glucose MonitoringDokument2 SeitenBlood Glucose MonitoringAnastasiafynnNoch keine Bewertungen
- CCS - Handbook of Anesthesiology (2005)Dokument180 SeitenCCS - Handbook of Anesthesiology (2005)Rojelle LezamaNoch keine Bewertungen
- Anaesthesia Resident's HandbookDokument445 SeitenAnaesthesia Resident's HandbookAnastasiafynn50% (2)
- Amino Acids Metabol Synth of UreaDokument32 SeitenAmino Acids Metabol Synth of UreaAnastasiafynn100% (1)
- Water and Sodium BalanceDokument4 SeitenWater and Sodium Balanceapi-3712326Noch keine Bewertungen
- AcidosisDokument2 SeitenAcidosisAnastasiafynnNoch keine Bewertungen