Beruflich Dokumente
Kultur Dokumente
PV NRT
Hochgeladen von
Khaleq Mohammad0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
85 Ansichten13 SeitenThe document provides examples of using stoichiometry to solve various gas, solution, and chemical reaction problems. It gives the balanced chemical equations and states the known and unknown quantities. The sample problems then show the step-by-step work to calculate the unknown value based on the mole and volume relationships in the balanced equation. This includes problems involving the relationships between moles and volumes of gases, masses and volumes of gases, and moles of reactants and products.
Originalbeschreibung:
Boyle's law
Originaltitel
pv=nrt
Copyright
© © All Rights Reserved
Verfügbare Formate
PPTX, PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenThe document provides examples of using stoichiometry to solve various gas, solution, and chemical reaction problems. It gives the balanced chemical equations and states the known and unknown quantities. The sample problems then show the step-by-step work to calculate the unknown value based on the mole and volume relationships in the balanced equation. This includes problems involving the relationships between moles and volumes of gases, masses and volumes of gases, and moles of reactants and products.
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PPTX, PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
85 Ansichten13 SeitenPV NRT
Hochgeladen von
Khaleq MohammadThe document provides examples of using stoichiometry to solve various gas, solution, and chemical reaction problems. It gives the balanced chemical equations and states the known and unknown quantities. The sample problems then show the step-by-step work to calculate the unknown value based on the mole and volume relationships in the balanced equation. This includes problems involving the relationships between moles and volumes of gases, masses and volumes of gases, and moles of reactants and products.
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PPTX, PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 13
UNIT 2: REVIEW
TIER 6
mbine the knowledge of gases and solutions to perform stoichiometric calculatio
Gas Stoichiometry
Sample Problem
Propane, C3H8, is a gas that is sometimes used as a fuel
for cooking and heating. The complete combustion of
propane occurs according to the following balanced
equation.
C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(g)
(a) What will be the volume, in liters, of oxygen
required for the complete combustion of 0.350 L of
propane?
(b) What will be the volume of carbon dioxide produced
in the reaction? Assume that all volume measurements
are made at the same temperature and pressure.
Gas Stoichiometry: VOLUME TO
VOLUME
Sample Problem Solution
Given: balanced chemical equation;
V of propane = 0.350 L
Unknown: V of O2
Solution: Because all volumes are to be
compared at
the same conditions, volume ratios can be
used like mole ratios.
.350L C3H8 X 3L CO2 = 1.05 L
CO2
1 L C3H8
Gas Stoichiometry:VOLUME TO MASS
Sample Problem
Calcium carbonate, CaCO3, also know as
limestone, can be heated to produce calcium
oxide (lime) and industrial chemical with a
wide variety of uses.
CaCO3 (s) CaO (s) + CO2(g)
How many grams of calcium carbonate must
be decomposed to produce 5.00L of carbon
dioxide gas at STP?
Sample Problem Solution
Given: - Balanced chemical equation;
CaCO3 (s) CaO (s) + CO2(g)
-V of carbon dioxide= 5.00 L
-STP conditions
Unknown: g of CaCO3
At STP 1 mole of gas occupies 22.4 L
5.00L x 1 mole CO2 = .223 moles CO2
22.4 L
.223 moles CO2 x 1 mole CaCO3 =.223 moles
CaCO3
1mole CO2
PROBLEM: How many liters of H2 gas at STP can be
produced by the reaction of 4.6 g Na and excess
water according to the following equation?
2Na(s) + 2 H2O(l) H2(g) + 2 NaOH (aq)
Sample Problem: MASS TO
VOLUME
Tungsten is produced by the
reaction of tungsten oxide with
hydrogen.
WO3 (s) + 3H2 (g) W(s) +3
H2O(l)
How many liter of hydrogen gas
at 35oC and .980 atm are needed
to react completely with 875 g of
Sample Problem Solution
Sample Problem Solution
Given: balanced chemical equation
WO3 (s) + 3H2 (g) W(s) + 3 H2O(l)
-P is .980 atm
-T is 35oC
-mass of WO3
Unknown: V of hydrogen gas
875 g WO3 x 3 mol H2 = 11.3 mole H2
231.84 g WO3
V = nRT = (11.3 mol)(.0821) (308K) = 292L
H2
P .980
PROBLEM: How many liters of gaseous carbon
monoxide at 27oC ad .247 atm can be produced from
the burning of 65.5g of carbon according to the
following equation?
2C(s) + O2(g) 2CO(g)
SOLUTION
STOICHIOMETRY
SAMPLE PROBLEM:
In a recent lab, a 2M hydrochloric acid was
reacted with 2.00 g of Magnesium.
Mg(s) + 2HCl MgCl2 (ag) + H2 (g)
(a)How many cm3 of hydrochloric acid would be
needed to react all the magnesium with out any
acid excess.
(b) How many dm3 of hydrogen gas could be
produced at STP
SAMPLE PROBLEM SOLUTION
GIVEN: Balanced equation: Mg(s) + 2HCl MgCl2 (ag) + H2 (g)
-mass of Mg =2.00g
-Molarity of HCl = 2mol dm-3
-STP conditions
UNKNOWN: (a) V of HCl solution
(b) V of H2 gas
(a) 2.00g Mg x 1mole Mg x 2 mole HCl x 1 dm3 HCl = .08 dm3
= 80 cm3
24.31g Mg 1 mole Mg 2 mol HCl
(b)2.00g Mg x 1 mole Mg x 1 mole H2 x 22.4 dm3 = 1.84
dm3
24.31 g Mg 1 mole Mg 1mole
PROBLEM: In a recent lab, a 3M nitric acid was
reacted with 3.50 g of zinc.
Zn(s) + 2HNO3 Zn(NO3 )2(ag) + H2 (g)
(a)How many cm3 of nitric acid would be needed to
react all the zinc with out any acid excess.
(b) How many dm3 of hydrogen gas could be
produced at STP
Das könnte Ihnen auch gefallen
- Chapter 12 - StoichiometryDokument50 SeitenChapter 12 - Stoichiometryapi-256257174Noch keine Bewertungen
- Gas StoichiometryDokument12 SeitenGas StoichiometryAnsel SotnasNoch keine Bewertungen
- Molaridad y NormalidadDokument4 SeitenMolaridad y NormalidadAlbert Stern100% (1)
- Gas StoichiometryDokument12 SeitenGas StoichiometryAhmed Ali SomosaNoch keine Bewertungen
- Stoichiometry - Analyzing Equations MathematicallyDokument17 SeitenStoichiometry - Analyzing Equations MathematicallyBrythanieNoch keine Bewertungen
- Chem ReviewDokument6 SeitenChem Reviewcoolio86Noch keine Bewertungen
- 2020-General Chemistry 1 Review WorksheetDokument4 Seiten2020-General Chemistry 1 Review WorksheetNgọc Thảo Vy NguyễnNoch keine Bewertungen
- LE2 ProbsetDokument5 SeitenLE2 ProbsetChris Andrew MendozaNoch keine Bewertungen
- 2010chem17 PracticeExercise1Dokument4 Seiten2010chem17 PracticeExercise1Erika Mae Adoja Espejo100% (1)
- LKM 3 Kel-2 Stoikio MetriDokument16 SeitenLKM 3 Kel-2 Stoikio MetriSalsabila AlmasNoch keine Bewertungen
- Chemchapter9answerkey 4Dokument8 SeitenChemchapter9answerkey 4jokerrr99900Noch keine Bewertungen
- ChytdvvhDokument10 SeitenChytdvvhFrancis TayagNoch keine Bewertungen
- Gas Laws Homework IIIDokument3 SeitenGas Laws Homework IIIchpwalkerNoch keine Bewertungen
- Effusion Diff and Gas Stoich Notes Outline AnswersDokument4 SeitenEffusion Diff and Gas Stoich Notes Outline Answersissa sherryNoch keine Bewertungen
- Chapter 9 ThermochemistryDokument6 SeitenChapter 9 ThermochemistryMohammad AfifNoch keine Bewertungen
- 110 WS Gas StoichiometryDokument2 Seiten110 WS Gas StoichiometryAnsel SotnasNoch keine Bewertungen
- 50 Chemistry Questions To Be Covered in Phase 2 (Master Tutors) - 2Dokument13 Seiten50 Chemistry Questions To Be Covered in Phase 2 (Master Tutors) - 2Chiluba EzronNoch keine Bewertungen
- As Unit 1 Chapter 1 Past PapersDokument20 SeitenAs Unit 1 Chapter 1 Past PapersK K Chamath Aachinthya0% (1)
- Stoichiometry: in Your TextbookDokument13 SeitenStoichiometry: in Your TextbookSaige RedNoch keine Bewertungen
- Ap Equilibrium WorksheetDokument5 SeitenAp Equilibrium Worksheetburcak gecNoch keine Bewertungen
- Revision Chapter 9-13Dokument123 SeitenRevision Chapter 9-13Ummul-KNoch keine Bewertungen
- Olimpiade Internasional Topik StoikiometriDokument7 SeitenOlimpiade Internasional Topik StoikiometriHeru Christian Strecker AritonangNoch keine Bewertungen
- N (G) - 2Nh (G) : StoichiometryDokument5 SeitenN (G) - 2Nh (G) : StoichiometryJaidenNoch keine Bewertungen
- Practice Questions For Test 2, Spring 2015Dokument10 SeitenPractice Questions For Test 2, Spring 2015Arianne Foster100% (1)
- Thermichemreview QuestionsDokument6 SeitenThermichemreview QuestionsSImiSaysRawrNoch keine Bewertungen
- Section 9: Reactions Involving GasesDokument6 SeitenSection 9: Reactions Involving GasesTravel UnlimitedNoch keine Bewertungen
- Thermochemistry Review Questions (Chemistry 30) : 2C H (l) + 15O (g) 12CO (g) + 6H O (l) ΔH = -6.535x10 kJDokument4 SeitenThermochemistry Review Questions (Chemistry 30) : 2C H (l) + 15O (g) 12CO (g) + 6H O (l) ΔH = -6.535x10 kJQuindo, Alexis FayeNoch keine Bewertungen
- Chapter 7 Chemical Energetics ExerciseDokument5 SeitenChapter 7 Chemical Energetics ExerciseAri Adiantari100% (1)
- Stoichiometry WorksheetDokument8 SeitenStoichiometry WorksheetdyannapandoraNoch keine Bewertungen
- EcDokument149 SeitenEcsurendar17_raj3406Noch keine Bewertungen
- 3.4 HomeworkDokument3 Seiten3.4 Homework허정우Noch keine Bewertungen
- Le Chatelier's - Common Ion EffectDokument13 SeitenLe Chatelier's - Common Ion EffectChenoa Sandhi C. SinghNoch keine Bewertungen
- CH 9 Packet KEYDokument5 SeitenCH 9 Packet KEYEvoli NatasNoch keine Bewertungen
- Gas StoichiometryDokument1 SeiteGas StoichiometryShdwplayerNoch keine Bewertungen
- Class15 Chemistry G11 Homework Dec 11-15Dokument4 SeitenClass15 Chemistry G11 Homework Dec 11-15ErinNoch keine Bewertungen
- C15PS3ADokument4 SeitenC15PS3ARoxanne de RoxasNoch keine Bewertungen
- Final Revision MCQ OrganicDokument7 SeitenFinal Revision MCQ Organiceeenus100% (1)
- Long Test ReviewerDokument15 SeitenLong Test ReviewerCaitlin OlayvarNoch keine Bewertungen
- Chemical Equilibrium Tutorial QuestionDokument4 SeitenChemical Equilibrium Tutorial QuestionHANIS HADIRAH BINTI HASHIMNoch keine Bewertungen
- DPP - 2 (Mole Concept)Dokument46 SeitenDPP - 2 (Mole Concept)Shourya ChandraNoch keine Bewertungen
- CPC 9Dokument8 SeitenCPC 9rajaraghuramvarmaNoch keine Bewertungen
- Probset 1Dokument2 SeitenProbset 1immaloginNoch keine Bewertungen
- Chapter3 Mole ConceptDokument10 SeitenChapter3 Mole Conceptmatyiman_123Noch keine Bewertungen
- Test, Bansal Chemicalequilibrium PDFDokument18 SeitenTest, Bansal Chemicalequilibrium PDFTarun Gupta0% (2)
- Chem 16 Lec - Sample Second Exam I. MULTIPLE CHOICE. Answer The Following by Writing The Best Answer From The ChoicesDokument6 SeitenChem 16 Lec - Sample Second Exam I. MULTIPLE CHOICE. Answer The Following by Writing The Best Answer From The ChoicesMaximillian LimNoch keine Bewertungen
- Istoichiometry WorksheetDokument10 SeitenIstoichiometry Worksheet11A-B03 Torres, Raphael Jose I.Noch keine Bewertungen
- Chapter 3Dokument13 SeitenChapter 3Siti Afiqah TajuddinNoch keine Bewertungen
- Y10 Moles Revision Questions AnswersDokument4 SeitenY10 Moles Revision Questions AnswersShougNoch keine Bewertungen
- Worksheet On Stoichiometry DIRECTIONS: Solve The Following Problems On Stoichiometry. Show Your WorkDokument2 SeitenWorksheet On Stoichiometry DIRECTIONS: Solve The Following Problems On Stoichiometry. Show Your WorkPrecious RabacNoch keine Bewertungen
- Chapter 9 Powerpoint Notes 2008Dokument39 SeitenChapter 9 Powerpoint Notes 2008Umar AsimNoch keine Bewertungen
- Tutorial 5 Equilibrium AnswerDokument4 SeitenTutorial 5 Equilibrium AnswerNor AishahNoch keine Bewertungen
- Calculations From Chemical Equations Part 1Dokument6 SeitenCalculations From Chemical Equations Part 1Daniel BerryNoch keine Bewertungen
- As LEVEL CalculationsDokument29 SeitenAs LEVEL CalculationsbuseNoch keine Bewertungen
- Chemical Equilibrium and K: Review Worksheet IDokument2 SeitenChemical Equilibrium and K: Review Worksheet ISachinNoch keine Bewertungen
- Gas StoichDokument5 SeitenGas StoichJarell De JuanNoch keine Bewertungen
- Gas StoichDokument5 SeitenGas StoichJarell De JuanNoch keine Bewertungen
- Mains Test 3Dokument7 SeitenMains Test 3SagarDalviNoch keine Bewertungen
- Practice Makes Perfect in Chemistry: Oxidation-ReductionVon EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionBewertung: 5 von 5 Sternen5/5 (1)
- Design of Sour Water Stripping System: February 2009Dokument23 SeitenDesign of Sour Water Stripping System: February 2009mohsen ranjbarNoch keine Bewertungen
- Motivational Interviewing (MI) Refers To ADokument5 SeitenMotivational Interviewing (MI) Refers To AJefri JohanesNoch keine Bewertungen
- Pathophysiology of Postpartum Hemorrhage and Third Stage of LaborDokument7 SeitenPathophysiology of Postpartum Hemorrhage and Third Stage of Labornouval_iqbalNoch keine Bewertungen
- digiPHONENT UG enDokument44 SeitendigiPHONENT UG enIrving Javier Leal OrtizNoch keine Bewertungen
- Vertico SynchroDokument16 SeitenVertico SynchrozpramasterNoch keine Bewertungen
- Anti Vawc PrimerDokument6 SeitenAnti Vawc PrimerCiddy Montemayor100% (1)
- Textile Chemical Brochure 8.6.22 - 031Dokument1 SeiteTextile Chemical Brochure 8.6.22 - 031NIKESH PRAKASHNoch keine Bewertungen
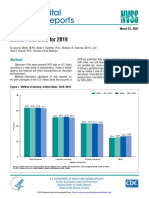
- National Vital Statistics Reports: Births: Final Data For 2019Dokument51 SeitenNational Vital Statistics Reports: Births: Final Data For 2019Simon ReichNoch keine Bewertungen
- CV TemplateDokument5 SeitenCV TemplateLopezDistrict FarmersHospitalNoch keine Bewertungen
- BMP (Class 14 - Class-17) WeldingDokument24 SeitenBMP (Class 14 - Class-17) WeldingAsesh PramanikNoch keine Bewertungen
- 2 5416087904969556847 PDFDokument480 Seiten2 5416087904969556847 PDFArvindhanNoch keine Bewertungen
- G1 CurvedDokument16 SeitenG1 CurvedElbert Ryan OcampoNoch keine Bewertungen
- 55 Gentle Ways To Take Care of Yourself When You're Busy Busy BusyDokument7 Seiten55 Gentle Ways To Take Care of Yourself When You're Busy Busy Busyvanjami100% (1)
- Finding Clara: Establishing The Biographical Details of Clara Peeters (Ca. 1587-After 1636)Dokument15 SeitenFinding Clara: Establishing The Biographical Details of Clara Peeters (Ca. 1587-After 1636)victoriagalapedroNoch keine Bewertungen
- Construction Regulations, 2014 PDFDokument58 SeitenConstruction Regulations, 2014 PDFbubele pamlaNoch keine Bewertungen
- PsychodramaDokument5 SeitenPsychodramaAkhila R KrishnaNoch keine Bewertungen
- DENSO Diagnostic TipsDokument1 SeiteDENSO Diagnostic TipsVerona MamaiaNoch keine Bewertungen
- Antibacterial Effects of Essential OilsDokument5 SeitenAntibacterial Effects of Essential Oilsnightshade.lorna100% (1)
- En CafDokument1 SeiteEn Caffareedee0% (1)
- Hygold 5000Bs: Base Oil Marketing SpecificationDokument1 SeiteHygold 5000Bs: Base Oil Marketing Specificationsamsoon80100% (1)
- The Zombie in The Brain and The Woman Who Died LaughingDokument40 SeitenThe Zombie in The Brain and The Woman Who Died Laughingcory_ruda100% (1)
- Pathology SEQ Answers - Adaptive Responses & Cell InjuryDokument7 SeitenPathology SEQ Answers - Adaptive Responses & Cell InjurysugandiNoch keine Bewertungen
- The Daily Star On 19.05.2021Dokument12 SeitenThe Daily Star On 19.05.2021nira miraNoch keine Bewertungen
- Unit: 3 - Vouching: by Mahitha VasanthiDokument15 SeitenUnit: 3 - Vouching: by Mahitha VasanthianuragNoch keine Bewertungen
- Material Safey Data Sheet: 1 Identification of SubstanceDokument6 SeitenMaterial Safey Data Sheet: 1 Identification of SubstanceRaihan MajumderNoch keine Bewertungen
- Measurement and Correlates of Family Caregiver Self-Efficacy For Managing DementiaDokument9 SeitenMeasurement and Correlates of Family Caregiver Self-Efficacy For Managing DementiariskhawatiNoch keine Bewertungen
- Civil Aviation Authority of BangladeshDokument1 SeiteCivil Aviation Authority of BangladeshS.M BadruzzamanNoch keine Bewertungen
- Amsoil Synthetic CVT Fluid (CVT)Dokument2 SeitenAmsoil Synthetic CVT Fluid (CVT)amsoildealerNoch keine Bewertungen
- SC4622 (CX) G3-399-04 - Ship Structural Access ManualDokument40 SeitenSC4622 (CX) G3-399-04 - Ship Structural Access ManualBen TanNoch keine Bewertungen
- Excavation PermitDokument2 SeitenExcavation PermitRajesh Kumar SinghNoch keine Bewertungen