Beruflich Dokumente
Kultur Dokumente
Co 2
Hochgeladen von
sri pragna0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
289 Ansichten23 SeitenThe document describes research into using carbon fiber composite molecular sieves (CFCMS) to remove CO2 from gas streams. CFCMS is composed of carbon fibers and phenolic resin binder that create an open, permeable structure suitable for adsorbing gases like CO2. Performance testing showed the material has a high adsorption capacity for CO2 and can be regenerated using electrical swing adsorption that disrupts attractive forces between CO2 and the carbon. Thermal conductivity measurements also indicated the material has anisotropic thermal properties suitable for gas separation applications.
Originalbeschreibung:
Co2 ppt
Originaltitel
Co2 ppt
Copyright
© © All Rights Reserved
Verfügbare Formate
PPTX, PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenThe document describes research into using carbon fiber composite molecular sieves (CFCMS) to remove CO2 from gas streams. CFCMS is composed of carbon fibers and phenolic resin binder that create an open, permeable structure suitable for adsorbing gases like CO2. Performance testing showed the material has a high adsorption capacity for CO2 and can be regenerated using electrical swing adsorption that disrupts attractive forces between CO2 and the carbon. Thermal conductivity measurements also indicated the material has anisotropic thermal properties suitable for gas separation applications.
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PPTX, PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
289 Ansichten23 SeitenCo 2
Hochgeladen von
sri pragnaThe document describes research into using carbon fiber composite molecular sieves (CFCMS) to remove CO2 from gas streams. CFCMS is composed of carbon fibers and phenolic resin binder that create an open, permeable structure suitable for adsorbing gases like CO2. Performance testing showed the material has a high adsorption capacity for CO2 and can be regenerated using electrical swing adsorption that disrupts attractive forces between CO2 and the carbon. Thermal conductivity measurements also indicated the material has anisotropic thermal properties suitable for gas separation applications.
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PPTX, PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 23
CO REMOVAL FROM GAS STREAMS BY
CARBON FIBER COMPOSITE MOLECULAR
SIEVE
presented by
:
VPS Pragna
A Supriya Devi
CONTENTS:
INTRODUCTION
PREPARATION OF CFCMS
PERFORMANCE CHARECTERISTICS
ELECTRONIC SWING ADSORPTION(ESA)
ADSORPTION AND SEPERATION DATA
THERMAL CONDUCTIVITY
CONCLUSION
INTRODUCTION
There is a great need for versatile,effective regenerable co2
separation.
An innovative technique known as CARBON FIBER COMPOSITE
MOLECULAR SIEVE is being investigated at OAK RIDGE
NATIONAL LABORATORY.
The CFCMS has distinct adsorption capacity, adsorption
kinetics advantages over conventional granular activated
carbons.
CFCMS
CFCMS is a monolithic carbon adsorbent material composed
of:
1.Petroleum derived isotropic pitch carbon fibers.
2.Phenolic resin derived carbon binder.
Adsorption of polar molecules and for some flat non- polar
molecules such as CO2 occurs on CFCMS in an effective
manner.
STRUCTURE OF CFCMS
The carbon fibers are bonded at their contact points creating
an open and permeable structure.
Macroscopic voids in the structure are approximately 50-
250ms,fibre lengths 50-1000ms,diameter 12-16ms.
High micro pore volumes :(0.5-1.0)cm3/gm.
BET surface area:(1000-2000)m2/kg.
PREPARATION OF CFCMS BY SLURRY PROCESS
PERFORMANCE CHARACTERISTICS
A gas flow loop was constructed to
determine and verify the performance
characteristics of CFCMS.
It has a capacity of about 50L/H and can
be operated up to 1000psi.
A side stream is lead from the loop and passes through a
adsorption containing the activated CFCMS.
The ESA sub system of the loop imparts the ability to
stimulate desorption of co2 from CFCMS sample.
ELECTRICAL SWING ADSORPTION(ESA)
The graph consists of three adsorption/desorption cycles.
first cycle-applied voltage and He purge gas.
second cycle-applied voltage and He purge gas.
third cycle-only He purge gas.
Hence the principle of an ESA system based upon the
electrical conductivity of CFCMS.
By passing electrical current attractive, static electrical or
vander waals forces between the carbon and adsorbate are
disrupted or reversed.
In an adsorption-desorption cycle, the CFCMS would be
saturated with co2 during the adsorption phase and
approximately 80% of co2 would be desorbed during
desorption phase.
ADSORPTION AND SEPARATION DATA
A high pressure adsorption
isotherms for CO2 and CH4
are given for two samples of
activated CFCMS as shown
below:
The Input gas switched to a 2:1 mixture of CO2 by CH4 at a
flow rate of 0.33slpm.
In the outlet stream, concentration of CH4 increases rapidly .
The adsorption of CO2 occurs and by the passage of low
voltage electric current ,desorbed gases were driven out by
the purge.
THERMAL CONDUCTIVITY
The thermal conductivity of CFCMS was determined by using
thermal flash technique.
The thermal diffusivity was calculated from thermal transient and
thermal conductivity was then determined
K=Cp
Where ,
=thermal diffusivity at test temperature.
=bulk density.
Cp=specific heat at test temperature.
Thermal conductivity of CFCMS is anisotropic.
CONCLUSIONS
The monolithic material has numerous advantages over
conventional power and granular activated carbons.
The ESA process is extremely fast in comparision to Pressure
Swing Apparatus(PSA) and Temperature Swing
Apparatus(TSA).
A systematic study of CFCMS to maximize its adsorption
capacity for C02 is needed.
Das könnte Ihnen auch gefallen
- Bubble Wake Dynamics in Liquids and Liquid-Solid SuspensionsVon EverandBubble Wake Dynamics in Liquids and Liquid-Solid SuspensionsNoch keine Bewertungen
- Lec 1Dokument27 SeitenLec 1Ihab OmarNoch keine Bewertungen
- Models of Imperfectly Mixed ReactorsDokument7 SeitenModels of Imperfectly Mixed ReactorsBlessy Gabayno100% (1)
- Turbulence: MIE1207 September, 2012Dokument128 SeitenTurbulence: MIE1207 September, 2012Sepehr SaNoch keine Bewertungen
- Membrane Gas-Solvent Contactor Trials of CO2 Absorption From SyngasDokument10 SeitenMembrane Gas-Solvent Contactor Trials of CO2 Absorption From SyngascurlychemNoch keine Bewertungen
- ANSYS Tutorial CFX Re-Meshing - EDRDokument12 SeitenANSYS Tutorial CFX Re-Meshing - EDRnes1b0Noch keine Bewertungen
- How To Minimise Scaleup DifficultiesDokument6 SeitenHow To Minimise Scaleup Difficultieskishore.charuNoch keine Bewertungen
- Flow ChemistryDokument6 SeitenFlow Chemistryrr1819Noch keine Bewertungen
- Introduction To ANSYS CFD Professional: Best Practice GuidelinesDokument11 SeitenIntroduction To ANSYS CFD Professional: Best Practice GuidelinesMahir SoyerNoch keine Bewertungen
- 06 Sparger PDFDokument17 Seiten06 Sparger PDFHafidho Ilham MNoch keine Bewertungen
- Pondash Vs Dry FlyashDokument8 SeitenPondash Vs Dry FlyashMere HamsafarNoch keine Bewertungen
- Chemical Process Safety: Kathmandu UniversityDokument16 SeitenChemical Process Safety: Kathmandu UniversityRojan PradhanNoch keine Bewertungen
- Chapter-3: Liquid Extraction (Solvent Extraction)Dokument69 SeitenChapter-3: Liquid Extraction (Solvent Extraction)Sanjay YadavNoch keine Bewertungen
- Phosgene Micro Reactor A I CheDokument9 SeitenPhosgene Micro Reactor A I CheJorge RamirezNoch keine Bewertungen
- Perry TabsDokument2 SeitenPerry TabsJILLIAN DALUPONoch keine Bewertungen
- MixDokument49 SeitenMixJoel TeslaNoch keine Bewertungen
- Inputs For Performance Mode: Maximum Throughput Case (9m Tower)Dokument18 SeitenInputs For Performance Mode: Maximum Throughput Case (9m Tower)Theodoros AtheridisNoch keine Bewertungen
- Transformer On Line MonitoringDokument6 SeitenTransformer On Line MonitoringSiva KumarNoch keine Bewertungen
- Chapter 11 - HeuristicsDokument40 SeitenChapter 11 - HeuristicsAmeerRashidNoch keine Bewertungen
- FGDDDDokument40 SeitenFGDDDMary Grace VelitarioNoch keine Bewertungen
- 12 PDO Heat TransferDokument55 Seiten12 PDO Heat Transferaxel2100Noch keine Bewertungen
- SpargerDokument14 SeitenSpargerNishevitha GangatharanNoch keine Bewertungen
- Gas - Liquid Reaction Engineering: University of DortmundDokument34 SeitenGas - Liquid Reaction Engineering: University of DortmundGirmaye Haile100% (2)
- Novel Method Gas Separation-PresentationDokument43 SeitenNovel Method Gas Separation-PresentationelelefanterozadoNoch keine Bewertungen
- Falling FilmDokument21 SeitenFalling FilmGhaya Bani RushaidNoch keine Bewertungen
- Influence of Stripper Operating Parameters On The Performance of Amine II - Vacuum Stippers PDFDokument10 SeitenInfluence of Stripper Operating Parameters On The Performance of Amine II - Vacuum Stippers PDFcargscribNoch keine Bewertungen
- Process UtilityDokument13 SeitenProcess UtilityAnupam Manoj100% (1)
- Difference Between Continuous and Batch ProcessDokument4 SeitenDifference Between Continuous and Batch ProcessPresupuesto 2020Noch keine Bewertungen
- Management of Scale Up of Adsorption in Fixed-Bed Column Systems - Odysseas KopsidasDokument45 SeitenManagement of Scale Up of Adsorption in Fixed-Bed Column Systems - Odysseas KopsidasΟδυσσεας ΚοψιδαςNoch keine Bewertungen
- Smart Monitoring and Power Factor Correction of Distribution Transformer Using IOTDokument5 SeitenSmart Monitoring and Power Factor Correction of Distribution Transformer Using IOTGRD JournalsNoch keine Bewertungen
- Rule of Thumb: Distillation and Gas AdsorptionDokument2 SeitenRule of Thumb: Distillation and Gas AdsorptionChristina Joana GuzmanNoch keine Bewertungen
- Scale UpDokument109 SeitenScale UpΟδυσσεας ΚοψιδαςNoch keine Bewertungen
- 0.-Rules of Thumb (Walas)Dokument7 Seiten0.-Rules of Thumb (Walas)Bryan PiguaveNoch keine Bewertungen
- Computational Fluid Dynamics (CFD) Blog - LEAP Australia & New Zealand - Turbulence Part 3 - Selection of Wall Functions and Y - To Best Capture The Turbulent Boundary LayerDokument7 SeitenComputational Fluid Dynamics (CFD) Blog - LEAP Australia & New Zealand - Turbulence Part 3 - Selection of Wall Functions and Y - To Best Capture The Turbulent Boundary LayerAbhiANoch keine Bewertungen
- 6 Crystallizer Design and Operation1Dokument22 Seiten6 Crystallizer Design and Operation1Shweta ChaudhariNoch keine Bewertungen
- CFD 2008 Quality Assurance of CFD Calculations - BPG by Thorsten HansenDokument46 SeitenCFD 2008 Quality Assurance of CFD Calculations - BPG by Thorsten HansenMoh SenNoch keine Bewertungen
- Effect of Mixing in Stirred Tank ReactorDokument33 SeitenEffect of Mixing in Stirred Tank ReactorRavi TejaNoch keine Bewertungen
- Agitated Vessel Heat TransferDokument7 SeitenAgitated Vessel Heat TransferalokbdasNoch keine Bewertungen
- PROC 5071: Process Equipment Design I: Mixing and AgitationDokument43 SeitenPROC 5071: Process Equipment Design I: Mixing and AgitationManoj BNoch keine Bewertungen
- Impact of Thermal Power Plant Fly Ash and Its Its Mitigation MesureDokument11 SeitenImpact of Thermal Power Plant Fly Ash and Its Its Mitigation MesureShamshad Ahmad100% (1)
- Cyclone Design Calculation Tool SimplifiedDokument6 SeitenCyclone Design Calculation Tool SimplifieddadNoch keine Bewertungen
- 39 Algal Oil Production 1Dokument21 Seiten39 Algal Oil Production 1Sai Srivathsava UdathuNoch keine Bewertungen
- Reaction CalorimetryDokument12 SeitenReaction CalorimetryMajeed KhanNoch keine Bewertungen
- Pressure DropDokument36 SeitenPressure DropdesignselvaNoch keine Bewertungen
- CFD Multiphase Modeling of Fluidized Bed Using Fluent SoftwareDokument44 SeitenCFD Multiphase Modeling of Fluidized Bed Using Fluent SoftwareAnonymous P8Bt46mk5INoch keine Bewertungen
- Chemical Engineering: Assistant Professor inDokument12 SeitenChemical Engineering: Assistant Professor inChemical EngineerNoch keine Bewertungen
- Chapter 5. ReactorsDokument22 SeitenChapter 5. ReactorsCheng Chiv ÏïNoch keine Bewertungen
- Ullmann Filtration FundamentalsDokument33 SeitenUllmann Filtration FundamentalspastorgeeNoch keine Bewertungen
- CALDENTEY, C., J. A Mechanistic Model For Liquid Hydrocyclones (LHC)Dokument109 SeitenCALDENTEY, C., J. A Mechanistic Model For Liquid Hydrocyclones (LHC)euderfilhoNoch keine Bewertungen
- Sequestering CO in The Built Environment: Calera CorporationDokument48 SeitenSequestering CO in The Built Environment: Calera CorporationPassmore DubeNoch keine Bewertungen
- Viscous Incompressible FlowDokument72 SeitenViscous Incompressible FlowEduardo LaloNoch keine Bewertungen
- Annular Condensation CFD Models For The Water-Steam in The Heat Pipe SystemsDokument9 SeitenAnnular Condensation CFD Models For The Water-Steam in The Heat Pipe SystemsRashed KaiserNoch keine Bewertungen
- Multiphase ReactorDokument37 SeitenMultiphase ReactorMaria Charlene Caraos TapiaNoch keine Bewertungen
- WWTPPT 161203034016Dokument30 SeitenWWTPPT 161203034016Lavish100% (1)
- Modeling and Simulation of CSTR For Manufacture of Propylene GlycolDokument6 SeitenModeling and Simulation of CSTR For Manufacture of Propylene Glycolantoojacome100% (1)
- Handbook of Thermal Conductivity, Volume 2: Organic Compounds C5 to C7Von EverandHandbook of Thermal Conductivity, Volume 2: Organic Compounds C5 to C7Noch keine Bewertungen
- High Pressure Phase Behaviour of Multicomponent Fluid MixturesVon EverandHigh Pressure Phase Behaviour of Multicomponent Fluid MixturesNoch keine Bewertungen
- Insights into Chemical Engineering: Selected Papers of P.V. DanckwertsVon EverandInsights into Chemical Engineering: Selected Papers of P.V. DanckwertsNoch keine Bewertungen
- Practical Chemical Thermodynamics for GeoscientistsVon EverandPractical Chemical Thermodynamics for GeoscientistsNoch keine Bewertungen
- Ekam Circles Training: Published On: 4 ContentDokument10 SeitenEkam Circles Training: Published On: 4 Contentsri pragnaNoch keine Bewertungen
- Assignment On Continuous Distillation - McCabe-Thiele Method (1) - 1442573024785Dokument5 SeitenAssignment On Continuous Distillation - McCabe-Thiele Method (1) - 1442573024785sri pragna0% (1)
- Sudheer CVDokument2 SeitenSudheer CVsri pragnaNoch keine Bewertungen
- FINALDokument9 SeitenFINALsri pragnaNoch keine Bewertungen
- Design of Packed Bed Column 2527Dokument19 SeitenDesign of Packed Bed Column 2527sri pragnaNoch keine Bewertungen
- RTD Studies in PFTRDokument6 SeitenRTD Studies in PFTRsri pragnaNoch keine Bewertungen
- B.Tech R13 PCE - 1453786111269Dokument219 SeitenB.Tech R13 PCE - 1453786111269sri pragnaNoch keine Bewertungen
- RTD Studies in CSTRDokument6 SeitenRTD Studies in CSTRsri pragnaNoch keine Bewertungen
- Plug Flow ReactorDokument7 SeitenPlug Flow Reactorsri pragnaNoch keine Bewertungen
- CHE 503 Agitation LiquidsDokument33 SeitenCHE 503 Agitation LiquidsNurtasha Atikah100% (1)
- Heat Transport by RadiationDokument19 SeitenHeat Transport by RadiationLuizaNoch keine Bewertungen
- CAL-ST-070!17!01 Rev01 Shipping Saddles CalculationDokument11 SeitenCAL-ST-070!17!01 Rev01 Shipping Saddles CalculationgiubelloNoch keine Bewertungen
- Analytical DynamicsDokument456 SeitenAnalytical DynamicsAykie Tropangzombie CastilloNoch keine Bewertungen
- A Summary of Essential Differences Between EC2 and BS8110 Code (2014 10 07)Dokument43 SeitenA Summary of Essential Differences Between EC2 and BS8110 Code (2014 10 07)tun20000Noch keine Bewertungen
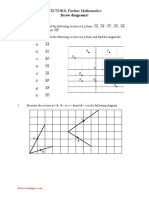
- OCR Further VectorsDokument13 SeitenOCR Further Vectorswill bellNoch keine Bewertungen
- Standard Sectional Dimension of SGP Pipe Steel Steel and ItsDokument3 SeitenStandard Sectional Dimension of SGP Pipe Steel Steel and Itsemut_m100% (1)
- 2nd 5Dokument4 Seiten2nd 5Le ScienceNoch keine Bewertungen
- Solutions: CBSE Final Exam. 2012Dokument19 SeitenSolutions: CBSE Final Exam. 2012Firdosh Khan100% (2)
- Thermochemistry: Prepared By: Ron Eric B. LegaspiDokument42 SeitenThermochemistry: Prepared By: Ron Eric B. LegaspiRon Eric Legaspi100% (1)
- The Electrical Conductivity of SolutionsDokument17 SeitenThe Electrical Conductivity of SolutionsHARIYANTO100% (1)
- Ultrasonic Characterization of Defects PDFDokument43 SeitenUltrasonic Characterization of Defects PDFFernandoiNoch keine Bewertungen
- 3 Forces and MotionDokument34 Seiten3 Forces and MotionIoryNoch keine Bewertungen
- Refresher QuestionsDokument33 SeitenRefresher QuestionsJoel James Gomez EvangelioNoch keine Bewertungen
- BS 499 PDFDokument36 SeitenBS 499 PDFkbldamNoch keine Bewertungen
- Vishal Chemistry Project CompleteDokument16 SeitenVishal Chemistry Project CompleteKislaya Mishra0% (1)
- Ashley P6 PPT 2Dokument4 SeitenAshley P6 PPT 2Reign AlfonsoNoch keine Bewertungen
- Sharjah Indian School, Sharjah. First Term Examination - June 2009Dokument3 SeitenSharjah Indian School, Sharjah. First Term Examination - June 2009ram parthsaNoch keine Bewertungen
- Violin LessonsDokument66 SeitenViolin LessonsKurt DuranNoch keine Bewertungen
- The Principle of Uncertainty - GamowDokument9 SeitenThe Principle of Uncertainty - GamowPPAANoch keine Bewertungen
- Creep Behaviour of Leaded BrassDokument4 SeitenCreep Behaviour of Leaded BrassAli MoussaNoch keine Bewertungen
- How To Solve Simultaneous Differential EquationsDokument5 SeitenHow To Solve Simultaneous Differential Equationsjin_phutaiNoch keine Bewertungen
- Horizontal Transport of Dredged Mixtures Part 1 - ENDokument65 SeitenHorizontal Transport of Dredged Mixtures Part 1 - ENedraket145Noch keine Bewertungen
- Questions & Answers: Astronomy (IOQA)Dokument9 SeitenQuestions & Answers: Astronomy (IOQA)Akshay TiwariNoch keine Bewertungen
- Pourbaix BasicsDokument16 SeitenPourbaix BasicsLaura DsbNoch keine Bewertungen
- Physics Study Material For FoundationDokument148 SeitenPhysics Study Material For FoundationApex Institute69% (13)
- Cracks in Concrete Core Due To Fire or Thermal Heating ShockDokument6 SeitenCracks in Concrete Core Due To Fire or Thermal Heating ShockaravindtankNoch keine Bewertungen
- Application of Spherical TrigonometryDokument17 SeitenApplication of Spherical TrigonometryAlfred Cyrus Redulfin100% (1)
- Technical SupportDokument2 SeitenTechnical SupportprabhakarNoch keine Bewertungen
- Baulieu, Laurent - Iliopoulos, Jean - Seneor, Roland-From Classical To Quantum Fields-Oxford University Press (2017)Dokument951 SeitenBaulieu, Laurent - Iliopoulos, Jean - Seneor, Roland-From Classical To Quantum Fields-Oxford University Press (2017)javier RendonNoch keine Bewertungen