Beruflich Dokumente
Kultur Dokumente
Organic Chemistry Basics at Warwick University
Hochgeladen von
Vina DwitaOriginalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Organic Chemistry Basics at Warwick University
Hochgeladen von
Vina DwitaCopyright:
Verfügbare Formate
Warwick University
Department of Chemistry
Year 1, Course CH158: Foundations of Chemistry
Section A3; Basics of Organic Chemistry
Professor Martin Wills
m.wills@warwick.ac.uk
Important: Please bear in mind that organic chemistry builds upon itself you must make sure
that you fully understand the earlier concepts before you move on to more challenging work.
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
1
Year 1 Foundation course, Section A3; Nomenclature of Organic Compounds
IUPAC has defined systematic rules for naming organic compounds.
These will have already been covered in detail at A-level and will
only be mentioned briefly here.
The naming system (and the resulting names) can become very long with complex
molecules, therefore this section will be restricted to simple compounds.
The IUPAC naming system involves the following components:
- Identification of major chain or ring
- Side chains and functional groups are added as
appropriate, in alphabetical order.
- The sums of numbers for substituents are minimised
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
2
Year 1 Foundation course, Section A3; Nomenclature of Organic Compounds
Examples:
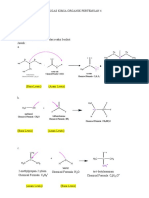
CH 3
H2 H2
C C CH CH 3
is 3-methyloctane,
H3C C C C
H2 H2 H2 not 5-methyloctane
2'
major chain 1' CH 3
H3C
CH
8 H2 H2
C 6 CH 4 C 2 CH 3
H3C H3C C CH C 1
CH 2 7 H2 5 3
H2 CH 3 CH 3 CH 3
H3C 6 CH 4 C 2 CH 3
C CH C 1 Is 5-(1-methylethyl)-2,2,4-trimethyloctane
7 H2 5 3
H2C CH 3 CH 3
CH 3
Is 4,5-diethyl-2,2-dimethylheptane
H2 H2
4 C 2 CH 3 4 C 2 CH 3
It is NOT H3C CH 1 H3C CH 1
3,4-diethyl-6,6-dimethylheptane! 3 3
OH Cl
Butan-2-ol 2-chlorobutane
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
3
Year 1 Foundation course, Section A3; Nomenclature of Organic Compounds
Many common names persist in organic chemistry, despite IUPAC rules, e.g.
Compound common name IUPAC name
O
C Acetone Propanone
H 3C CH 3
O
C Formaldehyde Methanal
H H
O
C Acetic acid Ethanoic acid
H 3C OH
O Dimethylether Methoxymethane
H 3C CH 3
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
4
Year 1 Foundation course, Section A3; Substitution level and functional groups
The substitution level of a carbon atom in an organic compound is determined by
the number of attached hydrogen atoms:
tertiary carbon (one H)
H3C CH 3
CH Primary C (3 hs)
H2 H2
C CH C CH 3
H3C C CH C
H2
CH 3 CH 3 CH 3
Secondary C (2 Hs) Quaternary C (0 Hs)
The rules differ for certain functional compounds e.g. alcohols:
H3C CH 3 H3C OH
H2 CH C
C
H3C OH OH CH 3 CH 3
Primary alcohol Secondary alcohol Tertiary alcohol
(2Hs on C attached to O) (1H on C attached to O) (0Hs on C attached to O)
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
5
Year 1 Foundation course, Section A3; Substitution level and functional groups
In the case of AMINES, the rules are different:
H H3C H
H3C H3C CH2
N N N
H CH 3 CH 3
Primary amine Secondary amine Tertiary amine
(2Hs on N) (1H on N) (0Hs on N)
Aromatic compounds: substitution position relative to group X
X para meta ortho
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
6
Year 1 Foundation course, Section A3; Substitution level and functional groups
Functional groups will be dealt with as they arise, however the following should be
committed to memory:
R= alkyl group, joining at C atom, e.g. CH3, c-C6H11, CH2CH2CH3 etc..
R H
R R C
R R
OH NH 2 SH Cl
O
Alcohol Amine Thiol Chloride Aldehyde
R OH R OR R R
R R
C C C
Br I
Iodide O O O
Bromide
Carboxylic acid Ester Ketone
R NH 2 R Cl R O R R H R O
C C C C C N
O O O O N O
R
Amide Acid Chloride Anhydride Imine Nitro
group
A cyclic ester is called a lactone, a cyclic amide a lactam
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
7
Year 1 Foundation course, Section A3; Line drawing - the standard from this point in the course
Line drawing represents an abbreviated shorthand representation of organic structures:
The rules are simple- Structures are written as a series of interconnected lines where each
apex is the position of a carbon atom. Heteroatoms (i.e. not H or C) are shown. H atoms are
not shown with the exception of those on heteroatoms.
Examples Full structure Abbreviated 'line-drawing' structure
H3C OH OH
Ethanol C
H2
H3C O O N.b. in some cases
Ethanal C the H atom of an
H aldehyde may be
H3C CH 2 illustrated
Propene C
H
H H
C C
Benzene
H C C H
C C
H H
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
8
Year 1 Foundation course, Section A3; Oxidation level
This is a useful tool for the understanding of organic reactions. It is slightly different
to the system used for the oxidation level of cations and anions.
In some cases it is obvious that a reaction is an oxidation or reduction, in other
cases they are not, for example:
H3C OH
C OH oxidation O
H3C O
H2 H3C
(removal of two H atoms) C
H
H3C CH 2
reduction
C H3C CH 3
H
(addition of two H atoms) C
H2
H3C CH 2 Oxidation or reduction? H2
C H3C C
H OH
(addition of O and C OH
H2
of two H atoms)
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
9
Year 1 Foundation course, Section A3; Oxidation level
To assign oxidation number (Nox), identify each each carbon atom that changes and assign oxidation
numbers as follows:
a) For each attached H assign -1.
b) For each attached heteroatom (O, N, S, Br, Cl, F, I etc.) assign +1.
c) Double or triple bonds to heteroatoms count double or triple respectively.
Then sum them for each molecule.
Example
Nox = -3 (3 attached H atoms)
H3C OH
Ethanol total -4
C
H2 Nox =-1 (1 attached O atom, 2 attached H atoms)
Nox = -3 (3 attached H atoms)
H3C O
Ethanal C total -2
H Nox = +1 (1 double bond to
O atom, 1 attached H atoms)
A change of +2 indicates an oxidation. A change of -2 indicates a reduction.
note + 2 or -2 is the typical change in oxidation level.
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
10
Year 1 Foundation course, Section A3; Molecular Stability - covalent vs ionic bonding
Many factors dictate the stability of atoms and ions. Hydrogen atoms gain stability if there are two
electrons in their electron shell. For first and second row elements, significant stability is derived
from an outer electronic configuration with 8 electrons.
Atoms can achieve this by i) gaining or losing electrons or ii) sharing them.
In the periodic table:
Central elements cannot
easily lose or gain electrons and
prefer to bond covalently. These are
H the most common atoms in organic compounds
Li Be B C N O F Ne row 1
Na Mg Al Si P S Cl Ar row 2
Atoms at the
extremes of the
rows can gain or lose an electron to
form an ionic lattice (e.g. NaCl)
The simplest example is where two hydrogen atoms combine to form H 2, with a covalent bond
between the atoms:
H . + H. H : H H H
Two H atoms, covalent bond- electrons shared
1 electron each.
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
11
Year 1 Foundation course, Section A3; Molecular Stability - covalent vs ionic bonding
Examples of covalent compounds:
(nb the three dimensional shapes of the molecules will be discussed in a later section)
Methane, CH4
H H
Combine 4 H atoms (1 outer electron ..
each) and 1 C atom (4 outer electrons) H : C : H H C H
to form methane: ..
H H
Ethane C2H6
H H H H
Combine 6 H atoms (1 outer electron .. ..
each) and 2 C atom (4 outer electrons) H : C :C : H H C C H
to form ethane .. ..
H H H H
Ethene C2H4
H H H
Combine 4 H atoms (1 outer electron H
: :
each) and 2 C atom (4 outer electrons) C :: C C C
to form ethen with double bond: .. ..
H H H H
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
12
Year 1 Foundation course, Section A3; Molecular Stability - covalent vs ionic bonding
Examples of covalent compounds:
Ethyne C2H2
Combine 2 H atoms (1 outer electron
each) and 2 C atom (4 outer electrons) H : C ::: C : H H C C H
to form ethyne with triple bond:
Methoxymethane (dimethylether) H H
.. .. H H
.. ..
Combine two C atoms (4 electrons), :
H C :O : C : H H C O C H
.. .. .. ..
one O atom (6 electrons) and six H H H H H
atoms (1 electron). Two pairs of electrons
(lone pairs) reside on the oxygen atom and the molecule has a 'bent' structure.
Methanal (formaldehyde)
H
Combine one C atom, one O atom .. .. H
..
C :: O : C O
and two H atoms with a C=O double .. ..
H H
bond. There are two lone pairs on O.
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
13
Year 1 Foundation course, Section A3; Molecular Stability - covalent vs ionic bonding
Examples of covalent compounds:
Ammonia NH3
H
Combine 3 H atoms (1 outer electron H
..
each) and 1 N atom (5 outer electrons) H : N : H N:
to form ammonia with lone pair on N ..
H H
The molecule has a tetrahedral shape.
Ammonium cation [NH4]+ - an example of when an overall charge is required for stability
H
Combine 4H atoms (1 outer electron H
..
each) and 1 N atom (5 outer electrons), H : N : H N H
then lose 1 electron to form ammonium .. H
H H
cation with an overall positive charge.
Same argument applies to protonated water - see if you can draw it.
More complex example: H
H H H
.. ..
Combine one boron (3 electrons),one N H : N : :H H N B H
and 6 H atoms to form a complex of .. B
..
H H H H
borane (BH3) and ammonia (NH3).
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
14
Year 1 Foundation course section A3; Molecules in 3D.
Linear combination of atomic orbital (LCAO) model.
Always remember that atomic orbitals (in atoms) combine to give molecular ones (in molecules - which is
obvious) but there are some rules:
i) n atomic orbitals form n molecular orbitals.
ii) The combination of atomic orbitals leads to the formation of a combination of bonding,
nonbonding and antibonding orbitals.
iii) In a stable molecule, the antibonding orbitals are empty, which is why it is stable!
e.g.
H . + H. H : H H H
The bond picture of dihydrogen formation:
Two H atoms, covalent bond- electrons shared
1 electron each.
This is what the orbitals do: Two molecular orbitals
Since only the bonding
H + H H H H H orbital is filled, the
molecule is stable.
Two atomic orbitals (s) bonding orbital antibonding orbital
1 electron in each one. low energy high energy
(, contains 2 electrons) (, empty)
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
15
Year 1 Foundation course section A3; Molecules in 3D.
Linear combination of atomic orbital (LCAO) model.
This is how the energy of the orbitals would be depicted:
Two molecular orbitals
H + H H H H H Since only the bonding
orbital is filled, the
Two atomic orbitals (s) bonding orbital antibonding orbital molecule is stable.
1 electron in each one. low energy high energy
(, contains 2 electrons) (, empty)
= one electron
antibonding orbital
Energy high energy,
H H
bonding orbital
low energy,
The electrons 'drop' into a lower energy position, which provides a driving force for the reaction, and stability.
Always bear this in mind when thinking about molecular orbital structure.
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
16
Year 1 Foundation course, Section A3; Bond Polarity
Covalency suggests equal sharing, but this is rarely the case because atoms differ in their
inherent ability to stabilise negative charge, I.e. their electronegativity. Electronegativity
increases in the direction of the arrows shown below (for the first two rows of the periodic table):
more
electronegative
H
Li Be B C N O F
Na Mg Al Si P S Cl
Pauling scale of electronegativity allows a quantitative comparison:
e.g. H (2.1), C (2.5), N (3.0), O (3.5), F (4.0), Cl (3.0), Br (2.8), I (2.5) etc.
As a result, most heteroatoms (X) are more electronegative that carbon and C-X bonds are
polarised so that there is a partial positive charge on the carbon atom.
The polarity See next page for examples
is illustrated thus: C X
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
17
Year 1 Foundation course, Section A3; Bond Polarity
Examples of covalent bonds which contain a dipole:
C Cl C Br C OH
Note: in the case of double and
triple bonds, resonance
C O C N (delocalisation) effects
also contribute to the polarity.
A few elements (notably metals) are less electronegative than C. As a result the dipole
is reversed:
C Si C Mg
This polarity effect is sometimes referred to as the INDUCTIVE effect, and operates through
sigma bonds in molecules (see a later section).
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
18
Year 1 Foundation course, Section A3; Formal Charge
Formal charge is a method for assigning charge to individual atoms in molecules. Although
it does not always give a perfect picture of true charge distribution, it is very helpful when
reaction mechanisms are being illustrated.
The definition of formal charge on a given (row 1 or 2) atom is as follows:
Formal charge on atom X (FC (X)) = (atomic group number of the atom* ignore transition
metals when counting!)-(number of bonds to the atom)- 2(number of lone pairs on the atom).
(You may see a slightly different version of the equation in other places).
Example:
Ethane -
H
for each (equivalent) C atom, FC(C)=4 -(4)-2(0) = 0
H
for each (equivalent) H atom, FC(C)=1 -(1)-2(0) = 0
C C H
H H Hence the formal charge on each atom in ethane is zero.
H
N.b - use a atomic group number of 1 for hydrogen.
* i.e. count from 1 to 8 across the row.
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
19
Year 1 Foundation course, Section A3; Formal Charge
Further examples:
Ethene -
H H for each (equivalent) C atom, FC(C)=4 -(4)-2(0) = 0
C C
for each (equivalent) H atom, FC(H)=1 -(1)-2(0) = 0
H H Hence the formal charge on each atom in ethene is zero.
Methoxymethane (remember this moelcule has two lone pairs on O).
.. for each (equivalent) C atom, FC(C)=4 -(4)-2(0) = 0
H H
O
H C .. C for each (equivalent) H atom, FC(H)=1 -(1)-2(0) = 0
H
for the O atom, FC(O)=6 -(2)-2(2) = 0
H H
Hence the formal charge on each atom is zero.
Ammonium cation (overall charge of +1)
for each (equivalent) H atom, Hence the formal H
H H
H FC(H)=1 -(1)-2(0) = 0 charge on the N H
N H for the N atom, atoms in the
FC(O)=5 -(4)-2(0) = +1 molecule is:. H
H
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
20
Year 1 Foundation course, Section A3; Formal Charge
Further examples:
Protonated water (overall charge of +1 and a lone pair on O)
for each (equivalent) H atom, Hence the formal H
H ..
FC(H)=1 -(1)-2(0) = 0 charge on the O H
O H for the O atom, atoms in the
H
H FC(O)=6 -(3)-2(1) = +1 molecule is:.
Borane-ammonia complex
H H for each (equivalent) H atom, H
Hence the formal H
FC(H)=1 -(1)-2(0) = 0
H N B H for the N atom, charge on the H N B H
H H FC(O)=5 -(4)-2(0) = +1 atoms in the
H H
for the B atom FC(B)=3-(4)-2(0) =-1 molecule is:.
Methyl cation (only 6 electrons around C): Tetrafluoroborate anion:
H F
H C
F B F
H
F
Use the formal charge definition to check the last two examples (n. b. there are three
lone pairs on each fluorine atom in BF4.
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
21
Year 1 Foundation course, Section A3; Acidity of organic compounds
Acidity is a measure of the ability of a compound to ionise to a proton and a negatively charged counterion.
Group. Organic compounds are not very acidic compared to strong mineral acids, however some are
stronger acids than others.
Lets put this into context.
The relative acidity in aqueous solution of a compound is defined by its pKa.
This is a measure of the inherent ability of any compound to lose a proton in an equilibrium process:
Ka pKa = - log [H ][RX ]
for HXR or - Log Ka
H + XR [HX]
Think about this for a second
If HXR is a strong acid, the equilibrium will be over to the right hand side. Ka will be high and
pKa will be a low number (possibly even negative). Carboxylic acids, the strongest organic
acids, have a pKa of around 5. If HXR is a weak acid he the equilibrium with be over to the left hand
side, Ka will be low and the pKa will be quite high. Alkanes (CnH2n+2) are very reluctant to lose a
proton and are weak acids. The pKa of an alkane is around 40. Most organic compounds have pKas
between these extremes.
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
22
Year 1 Foundation course, Section A3; Acidity of organic compounds
Nb - a related scale, pH, is a measure of the amount of protons in a solution at any moment.
pH is defined as -log [H+].
Here are a few more examples of pKa values of organic compounds.
Remember that each unit of pKa represents a tenfold change in acidity.
Some examples (no. relates to circled proton) are given below:
compound pKa compound pKa
alkane H 40 16
alcohol O H
amine N H 30
phenol 10
O H
O
O
H 20
ketone O H 5
carboxylic
acid
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
23
Year 1 Foundation course section A3; Molecules in 3D.
Rehybridisation and VSEPR:
The three-dimensional structure of organic compounds often influences their properties and reactivity.
Each carbon atom in an organic molecule can be linked to four, three or two other groups. In each case
the orbital structure and three-dimension shape around that carbon atom is different
In the case of a carbon atom attached to four other groups by single bonds, the single 2s and the three
2p orbitals gain stability by mixing (rehybridisation) to form four sp3 orbitals. These are all arranged
at mutual 109.5 degree angles to each other and define a tetrahedral shape:
3 x 2p on a carbon atom combine to form 4 x sp3:
1 x 2s
H
C H
which lie at mutual 109.5 degrees H C
in a molecule such as methane, CH4: H H
H
H
H
A tetrahedral shape is favoured because this maximises the distance between the filled orbitals, which
contain negatively charged electrons, and therefore repel each other. This is known as the valence shell
electron pair repulsion (or VSEPR), and often dominates the shape of molecules.
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
24
Year 1 Foundation course section A3; Molecules in 3D.
The VSEPR model for the structure of molecules also explains why molecules such as ammonia and
water are not flat or linear respectively. Their structures are bent because of repulsion effect of the
electrons in the lone pairs (which are in sp 3 orbitals).
At the nitrogen atom in ammonia, NH3:
1 x 2s 3 x 2p on a nitrogen atom combine to form 4 x sp3: orbitals
lone pair N
..
which lie at mutual 109.5 degrees N As a result, ammonia is tetrahedral
H H and has a significant dipole.
in the ammonia molecule, NH3: H + H
H + +
H
At the oxygen atom in water, H2O:
1 x 2s 3 x 2p on an oxygen atom combine to form 4 x sp3: orbitals
lone pair O
..
As a result, water is tetrahedral
O
which lie at mutual 109.5 degrees + H : and has a significant dipole.
in the ammonia molecule, NH3: H H
+
H lone pair
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
25
Year 1 Foundation course section A3; Molecules in 3D.
Some things to be aware of:
i) Symmetrical, tetrahedral, compounds have no overall dipole:
H +
The shape is still tetrahedral, and eacn N-H bond has a
For example in the ammonium cation, NH4+
N dipole, but there is no overall dipole, because they
H H cancel out.
+ H
+ +
H
For the same reason, methane has no overall dipole either: C
H H
H
ii) Molecules which are electron deficient, such as borane (BH3), retain a trigonal shape. Why?
Well, without an electron pair, there is nothing to repel with!!!
Borane, BH3: (one B atom with three electrons H H
H B H and three H atoms with one electron :
H :
each form borane, which has only 6 B H :B H
o electrons at the B atom:
Flat (trigonal), 120 bond angles H H
no overall dipole
B
H H
In contrast, borohydride anion,
NH4-, is tetrahedral, with no overall dipole: H
B
H H (3 sp2 orbitals and one p orbital,
H orthogonal to plan of BH3 atoms)
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
26
Year 1 Foundation course section A3; Molecules in 3D
In the case of a carbon atom attached to three other groups (by two single bonds and one double bond)
the single 2s and two2p orbitals mix (rehybridise) to form three sp 2 orbitals. These are all arranged at
mutual 120 degree angles to each other and define a trigonal shape, the remaining p orbital projects out
of the plane of the three sp2 orbitals and overlaps with an identical orbital on an adjacent atom
to form the double bond:
2 x 2p on a carbon atom combine to form 3 x sp2 orbitals:
1 x 2s
which lie at mutual 120 degrees H
in a molecule such as etheneC2H6, H H H
whilst the remaining p orbital C C
H H
forms the double bond:
H H
The resulting structure is rigid and cannot rotate about the C=C bond without breakage of the
bond between the p-orbitals (the p bond).
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
27
Year 1 Foundation course section A3; Molecules in 3D
In the case of a carbon atom attached to two other groups (by one single bonds and one triple bond)
the single 2s and one 2p orbitals mix (rehybridise) to form two sp orbitals. These are all arranged at
mutual 180 degree angles to each other and define a linear shape, the remaining p orbitals projecting out
from the sp orbital to overlap with identical orbitals on an adjacent atom to form the triple bond:
1 x 2s 1 x 2p on a carbon atom combine to form 2 x sp2 orbitals:
which lie at 180 degrees
in a molecule such as ethyneC2H4,
whilst the remaining p orbitals H H C C H
H
form the triple bond:
Rehybridisation of orbitals of this type is not limited to carbon, of course. Many other row 1 and 2
atoms (notably N) can rehybridise within organic molecules.
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
28
Year 1 Foundation course section A3; Molecules in 3D Conformation and configuration
Configuration is a fixed stereochemical property of compounds. Unlike conformation, a change
in configuration requires bonds to be broken and formed. Any molecule has a limited number of
configurations in which it can exist.
Alkenes can exist in two configurations, for example but-2-ene may have the terminal methyl groups
in a trans (across from each other) or cis (on the same side) position:
H3C H H3C CH 3
trans-but-2-ene C C cis but-2-ene C C
H CH 3 H H
Changing trans butadiene into cis- butadiene (or vice versa) requires the breaking, and
subsequent reforming, of the p bond. This is a high- energy process and does not take place
at room temperature. At room temperature, but-2-ene (and other alkenes) can be physically
separated into the two pure isomers.
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
29
Year 1 Foundation course section A3; Molecules in 3D - Conformation and configuration
The configuration of an alkene can be obvious in some cases (such as but-2-ene) however in others
it is not, for example is the molecule below a cis or trans alkene?
H3C H2C CH 3
C C
H CH 3
In order to provide an unambiguous means for assigning configuration to alkenes (and also to
chiral centres as you will see later), organic chemists have adopted the Cahn-Ingold-Prelog (CIP)
rules for configurational assignment.
These are simple to use - first one assigns a priority to each group attached to each carbon atom at
each end of the alkene. I will describe to priority rules in the next slide. We then define the alkene as
either Z (from the German zusammen, together) or E (from the German entgegen, across):
High Low High
High
Z alkene: C C E alkene: C C
Low Low High Low
(relative priorities are of a group on a C atom to its partner on the same atom)
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
30
Year 1 Foundation course section A3; Molecules in 3D Conformation and configuration
The CIP priority rules are defined as follows, in their own order of priority:
a) Atoms of higher atomic number have priority:
H3C CH3 High High
e.g. C C C C
H H H H
In this molecule the attached carbon atoms at each end of the double bond
have priority over the attached H atoms, hence this is a Z alkene
b) When the attached atoms are identical on each side, isotopes of higher mass have priority
H3C D High High
e.g. C C C C
H H H H
In this molecule the attached carbon and deuterium (deuterium is the H-2 isotope)
atoms at each end of the double bond have priority over the attached H atoms, hence
this is a Z alkene
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
31
Year 1 Foundation course section A3; Molecules in 3D - Conformation and configuration
The CIP priority rules are defined as follows, in their own order of priority:
a) When the atoms and isotopes attached on each side are identical, move out until a point of
difference is encountered and apply the following rules:
a) Priority goes to the group with the element of highest atomic number at the point of difference.
H2C NH 2 Low High
H
e.g. C C C C
H3C C CH 3 High Low
H3C CH 3 E alkene
b) Priority goes to the group with the highest sum of atomic numbers if the atoms are of the
same types at the point of difference. In the example below, the point of difference on the right hand
side is two carbons away from the alkene carbon atom
CH 3
H H2C CH Low High
CH 3
e.g. C C C C
H3C H2C CH 2 High Low
H2 E alkene
H2C C
CH 3
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
32
Year 1 Foundation course section A3; Molecules in 3D - Conformation and configuration
This is how I worked out the last example (right hand side only):
Imagine you are moving out from the C tothe adjacent atoms. Going both up (to C1) and down
(to C1') leads to a CH2 group, i.e. no difference. Moving to the next atom reveals that C2 (top
branch) is attached to C,C,H but that C2' (lower branch) is attached to C,H,H. The upper branch has
the highest sum of attached atomic numbers and therefore has priority.
1 2 CH 3
H H2C CH
CH 3 Upper: C-> C1(C,H,H) -> C2(C,C,H) Low High
C C 2' C C
H3C H2C CH 2
H2 Lower: C-> C1(C,H,H) -> C2(C,H,H) High Low
1' H2C C E alkene
CH 3
There is one more rule:
d) In the case of double and triple bonds, dummy atoms should be added and counted in the
determination of priority. See next slide.
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
33
Year 1 Foundation course section A3; Molecules in 3D - Conformation and configuration
Here is an example of the determination of configuration for an alkene attached
to a double bond:
C C <--dummy atoms
1 2
1 2
H CH CH 2 Upper: C-> C1(C,C,H)
H HC CH 2
C C 2'
C C 2'
H3C H2C CH 2
H3C H2C CH 2 H2 Lower: C-> C1(C,H,H)
H2 1' H2C C
1' H2C C
CH 3
CH 3
Low High
C C
High Low
E alkene
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
34
Year 1 Foundation course section A3; Molecules in 3D - Conformation and configuration
CIP priority rules are also applied to the determination of configuration at chiral centres (a chiral
molecule is one which is not superimposable on its mirror image, rather like your hands). The simplest
form of a chiral centre is one with a carbon atom attached to four different groups.
mirror
E.g.
CBrClFH is F
F
a chiral molecule Cl
Cl
the two forms (known C C
as enantiomers) can Br Br
be illustrated thus: H H
To assign a configuration to a chiral molecule such as the one shown above we first assign
CIP priorities to all four groups using the same rules:
e.g. F 3
Cl 2 1 = highest priority
C C
to 4 = lowest priority.
Br 1 4
H
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
35
Year 1 Foundation course section A3; Molecules in 3D - Conformation and configuration
We then view the molecule, with the assigned priorities, along the C-4 bond (with the 4 behind the central
carbon atom. Finally, draw an arrow from atom with priority 1 to priority 2 to priority 3 in turn:
3 2 3
2 atom 4 is behind C
C C
1 4 1
In this case the arrow is clockwise; this is therefore referred to as a R isomer (R comes from the Latin
rectus, for right). Isomers of this type are sometimes called enantiomers.
F
Cl R enantiomer
C
Br
H
The mirror image of the molecule above is the S enantiomer (from the Latin sinister for left)
F 3
Cl 2 3 2
C C S enantiomer
C 1
Br 4
H 1
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
36
Year 1 Foundation course section A3; Molecules in 3D - Conformation and configuration
Here are a couple of examples - can you see the derivation of the configuration?
3
H CH 3 4 3
R configuration
H2N SH
2 1 2 1
3
H CH 3 4 3
S configuration
H2N
SH 1 2 1 2
One carbon atom makes all the difference!
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
37
Year 1 Foundation course section A3; Molecules in 3D - Conformation and configuration
This is quite important please read it.
Some other conventions are used to defined the configuration at chiral centres e.g.
l - molecule with a negative optical rotation (from the Greek for levorotatory; left)
d - molecule with a positive optical rotation (from the Greek for dextrarotatory; right)
The D/L notation ( a very old convention) is derived from the signs of optical rotation of R and S
glyceraldehyde respectively:
H OH HO H
HO HO
CHO CHO
D (+)-glyceraldehyde (also R) L (-)-glyceraldehyde (also S)
The trivial convention for the absolute configurations of sugars derives from the D/L notation above.
D-glucose is the natural enantiomer (costs 20/kg) whilst L-glucose is very rare (31/g!).
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
38
Year 1 Foundation course section A3; Molecules in 3D - Conformation and configuration
Amino acids are classified into L- (natural) and D- (unnatural)
H R R H
H2N CO2H H2N CO2H
L - amino acid D- amino acid
Most L-amino acids are of S- configuration.
Despite all the different notations, R and S is the one YOU should learn how to use.
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
39
Year 1 Foundation course section A3; Molecules in 3D - Conformation and configuration
The two mirror-images of chiral compounds can have dramatically different physical properties.
That is because we ourselves are made up of molecules of one handedness. Try assigning R/S to these:
O O
O NHiPr O NHiPr
CO 2H CO 2H
OH OH N O N O
NH NH
HS NH 2 HS NH 2 O O
Propranolol: Penicillamine Thalidomide
R is a heart drug R is an antiarthritic R is mutagenic
S is a contraceptive S is a highly toxic S is anti-emetic
Et O Et O
Cl Cl
CO 2H CO 2H
N N
O O
Et Et
Limonene:
'Dual' Ibuprofen
R has a lemon odour
R is an herbicide R is anti-inflammatory
S has an orange odour
S is a pesticide S is inactive
Professor M. Wills CH158 Year 1 A3 Basics of Organic Chemistry
40
Das könnte Ihnen auch gefallen
- Basic Organic Chemistry and Mechanisms Revision From M Wills For When You Are Lost and ConfusedDokument128 SeitenBasic Organic Chemistry and Mechanisms Revision From M Wills For When You Are Lost and ConfusedJasim Al-rufayeNoch keine Bewertungen
- Hidro KarbonDokument43 SeitenHidro KarbonElisabet NoviantiNoch keine Bewertungen
- Alkanes: 1.1 Classification of HydrocarbonDokument33 SeitenAlkanes: 1.1 Classification of HydrocarbonKhizra TehreemNoch keine Bewertungen
- Aldehydes and KetonesDokument58 SeitenAldehydes and KetonesAniruddha KawadeNoch keine Bewertungen
- AldeDokument2 SeitenAlde14.Hajjan MNoch keine Bewertungen
- Naming Alcohols PDFDokument1 SeiteNaming Alcohols PDFumairgul841Noch keine Bewertungen
- 4 AllDokument28 Seiten4 AllEtwo Xiao0% (1)
- Organic Chemistry Worksheet for Defence University College of Health ScienceDokument4 SeitenOrganic Chemistry Worksheet for Defence University College of Health ScienceKerala MekuriyaNoch keine Bewertungen
- Pentene: CydohexadieneDokument4 SeitenPentene: CydohexadieneNat PanupongNoch keine Bewertungen
- Chemicals Used As Disinfectants - Active Ingredients and Enhancing Additives PDFDokument18 SeitenChemicals Used As Disinfectants - Active Ingredients and Enhancing Additives PDFjayNoch keine Bewertungen
- Chemicals Used & Modes of Actions of DisinfectantsDokument25 SeitenChemicals Used & Modes of Actions of DisinfectantsjayNoch keine Bewertungen
- Chemistry Ii (Ecf 0024) : Tutorial 4Dokument3 SeitenChemistry Ii (Ecf 0024) : Tutorial 4utpNoch keine Bewertungen
- 2022 Intro To Org Chem Tutorial Section B AnsDokument17 Seiten2022 Intro To Org Chem Tutorial Section B AnsLow Jia YingNoch keine Bewertungen
- MATERI ALKANA Insyaallah FixDokument2 SeitenMATERI ALKANA Insyaallah Fixikfisaini yuniarNoch keine Bewertungen
- PS 3Dokument3 SeitenPS 3Denisse Leonoras-PatersonNoch keine Bewertungen
- i) 2,2-Dimethylbutane(ii) 3-Ethyl-2-methylhexane (iii) 2-Methyl-5-ethylnonaneDokument45 Seiteni) 2,2-Dimethylbutane(ii) 3-Ethyl-2-methylhexane (iii) 2-Methyl-5-ethylnonaneAnubhab100% (2)
- Naming of Alkynes Based On IUPAC Rules: CH H C CH CHDokument3 SeitenNaming of Alkynes Based On IUPAC Rules: CH H C CH CHikfisaini yuniarNoch keine Bewertungen
- Organic Chemistry 1&2Dokument142 SeitenOrganic Chemistry 1&2Kennedy ChitayiNoch keine Bewertungen
- Elimination ReactionDokument6 SeitenElimination ReactionDxng 1Noch keine Bewertungen
- Organic Nomenclature Bank 1Dokument18 SeitenOrganic Nomenclature Bank 1talaaty599Noch keine Bewertungen
- II. ASAM KARBOKSILATDokument13 SeitenII. ASAM KARBOKSILATFaykar RezaNoch keine Bewertungen
- Cataliza Enzime Si ProteineDokument10 SeitenCataliza Enzime Si Proteineseranim22Noch keine Bewertungen
- AldehidosDokument1 SeiteAldehidosJuan Sebastian AlarconNoch keine Bewertungen
- Organic Chemistry 1 - Chem - f3 - v1 1Dokument77 SeitenOrganic Chemistry 1 - Chem - f3 - v1 1Lubanga N JamesNoch keine Bewertungen
- Organic NomenclatureDokument12 SeitenOrganic Nomenclatureitbwng100% (1)
- Exercise C7 - Ans SchemeDokument3 SeitenExercise C7 - Ans Schemeknn233610437Noch keine Bewertungen
- 11.2 Organic-Nomenclature Practice 2Dokument4 Seiten11.2 Organic-Nomenclature Practice 2Laura LiuNoch keine Bewertungen
- Nomenclature of Organic CompoundsDokument80 SeitenNomenclature of Organic CompoundsSajjad MiraniNoch keine Bewertungen
- Chapter 3 - Wade - 9th Ed - 1Dokument79 SeitenChapter 3 - Wade - 9th Ed - 1lizNoch keine Bewertungen
- Problem 26 Chemical Structure and Absolute Stereochemistry of ConiineDokument6 SeitenProblem 26 Chemical Structure and Absolute Stereochemistry of ConiineLê Hoàng MinhNoch keine Bewertungen
- Wa0005Dokument5 SeitenWa0005rishigullipalli2007Noch keine Bewertungen
- IUPAC - Class 11Dokument32 SeitenIUPAC - Class 11NitishNoch keine Bewertungen
- LKPD FixDokument6 SeitenLKPD FixNirmala DelaNoch keine Bewertungen
- 9.0 Carbonyl 2022 (Lecturer)Dokument13 Seiten9.0 Carbonyl 2022 (Lecturer)naderaqistina23Noch keine Bewertungen
- 2023 Alkanes Tutorial Ans SchemeDokument14 Seiten2023 Alkanes Tutorial Ans SchemeJun JieNoch keine Bewertungen
- Nomenclature and Introduction of Major Functional Groups - 2011Dokument316 SeitenNomenclature and Introduction of Major Functional Groups - 2011Hesham El-RoussassiNoch keine Bewertungen
- CH 2 (Crude Oil Characterisation) 2018 BDokument63 SeitenCH 2 (Crude Oil Characterisation) 2018 Bnafisa afariNoch keine Bewertungen
- Organic Nomenclature 4Dokument4 SeitenOrganic Nomenclature 4kayo lumasyNoch keine Bewertungen
- Alkenes (Fasi)Dokument3 SeitenAlkenes (Fasi)kjjkimkmkNoch keine Bewertungen
- Hydrocarbons: 1-6-2021 Organic 1 - Introducton - Dr. Ahmed M El-Morsy 1Dokument14 SeitenHydrocarbons: 1-6-2021 Organic 1 - Introducton - Dr. Ahmed M El-Morsy 1Hasen umerNoch keine Bewertungen
- 2010 Organic Chemistry Assignment 1 AnswersDokument6 Seiten2010 Organic Chemistry Assignment 1 Answersvokasa4037Noch keine Bewertungen
- Chapter 2-AlkanesDokument63 SeitenChapter 2-AlkanesNURUL BALQIS DZULKIFLINoch keine Bewertungen
- Solution Manual For Organic and Biochemistry For Today 8th Edition Seager Slabaugh 1133605141 9781133605140Dokument36 SeitenSolution Manual For Organic and Biochemistry For Today 8th Edition Seager Slabaugh 1133605141 9781133605140manuelfloydmnejszfdwg100% (22)
- Solutions Manual Chapter22Dokument62 SeitenSolutions Manual Chapter22더브레인코어과학관Noch keine Bewertungen
- Zumdahl Chemprin 6e CSM ch21 PDFDokument63 SeitenZumdahl Chemprin 6e CSM ch21 PDFMohit ShirpurkarNoch keine Bewertungen
- Amino Acids and Peptide BondDokument61 SeitenAmino Acids and Peptide Bondranger cleetusNoch keine Bewertungen
- Nomenclature of Carbon CompoundDokument12 SeitenNomenclature of Carbon CompoundTj NovalNoch keine Bewertungen
- Hydrocarbons WorksheetDokument3 SeitenHydrocarbons WorksheetDhruti AsharNoch keine Bewertungen
- Define Chemistry Terms, Organic Compounds, and HybridizationDokument7 SeitenDefine Chemistry Terms, Organic Compounds, and HybridizationKerala MekuriyaNoch keine Bewertungen
- 5 JeeDokument14 Seiten5 JeeYaswanth PedapudiNoch keine Bewertungen
- Aminoglycosides & Spectinomycin: Daniel H. Deck, Pharmd & Lisa G. Winston, MDDokument4 SeitenAminoglycosides & Spectinomycin: Daniel H. Deck, Pharmd & Lisa G. Winston, MDVân BùiNoch keine Bewertungen
- Tutorial 3 PDFDokument1 SeiteTutorial 3 PDFAmirahNoch keine Bewertungen
- Nomenclature in Organic CompoundsDokument159 SeitenNomenclature in Organic CompoundsPrecious AjiboyeNoch keine Bewertungen
- Ups3 q2-q6 Supianazma EditDokument5 SeitenUps3 q2-q6 Supianazma EditSupia NazmaNoch keine Bewertungen
- đọc tênDokument7 Seitenđọc tênthái đức thắngNoch keine Bewertungen
- F H C B F F F Chemical Formula: CH BF ODokument3 SeitenF H C B F F F Chemical Formula: CH BF OFadilla AzhariNoch keine Bewertungen
- 11 - Alcohol Ethers Thiols Wks KeyDokument5 Seiten11 - Alcohol Ethers Thiols Wks KeyMaria Aira Mendoza100% (1)
- 6carboxylic Acids PDFDokument29 Seiten6carboxylic Acids PDFsharmimiameerasanadyNoch keine Bewertungen
- Chapter 16 SmithDokument26 SeitenChapter 16 SmithSandipan SahaNoch keine Bewertungen
- Just A...Dokument2 SeitenJust A...Vina DwitaNoch keine Bewertungen
- Otk 2gfgDokument1 SeiteOtk 2gfgVina DwitaNoch keine Bewertungen
- Presentation 1Dokument1 SeitePresentation 1Vina DwitaNoch keine Bewertungen
- Linear regression analysis of pH vs log MR/HMRDokument2 SeitenLinear regression analysis of pH vs log MR/HMRVina DwitaNoch keine Bewertungen
- Basic Principle & 1st LawDokument43 SeitenBasic Principle & 1st LawVina DwitaNoch keine Bewertungen
- ServoDokument1 SeiteServoVina DwitaNoch keine Bewertungen
- Tetapan Laju Reaksi Dan Energi AktivasiDokument17 SeitenTetapan Laju Reaksi Dan Energi AktivasibetyNoch keine Bewertungen
- Thermodynamic Relations DiagramDokument23 SeitenThermodynamic Relations DiagramVina Dwita100% (1)
- Power Production & RefrigerationDokument30 SeitenPower Production & RefrigerationVina Dwita100% (1)
- LirikDokument7 SeitenLirikVina DwitaNoch keine Bewertungen
- LirikDokument7 SeitenLirikVina DwitaNoch keine Bewertungen
- Solution Manual For Chemistry The Central Science 13 e 13th EditionDokument17 SeitenSolution Manual For Chemistry The Central Science 13 e 13th EditionAndrewMartinezjrqo100% (38)
- LIPID ANALYSISDokument20 SeitenLIPID ANALYSIS3amabelle arevalo100% (2)
- NAT Reviewer 2 PHYSICAL SCIENCEDokument33 SeitenNAT Reviewer 2 PHYSICAL SCIENCEChary Johanne Meneses100% (1)
- Buhari, A. (2014, January 25) Science Revision. SlideshareDokument7 SeitenBuhari, A. (2014, January 25) Science Revision. SlideshareHannah AlfonsoNoch keine Bewertungen
- Investigation of The Chemical and Physical Properties of Ionic and Covalent BondingDokument7 SeitenInvestigation of The Chemical and Physical Properties of Ionic and Covalent Bondingapi-238781118100% (1)
- Are A Class of Organic Compounds That Have An OxygenDokument6 SeitenAre A Class of Organic Compounds That Have An OxygenArianne MontañoNoch keine Bewertungen
- Laboratory Activity No. 19 Data SheetDokument5 SeitenLaboratory Activity No. 19 Data SheetFreddy MarsucNoch keine Bewertungen
- Describe The Valence Shell Electron Pair Repulsion (VSEPR) TheoryDokument4 SeitenDescribe The Valence Shell Electron Pair Repulsion (VSEPR) TheoryhadassahhadidNoch keine Bewertungen
- Grade 11 ChemistryDokument17 SeitenGrade 11 ChemistryKevin George100% (1)
- Chemistry States of MatterDokument83 SeitenChemistry States of MatterEnzo ValendinoNoch keine Bewertungen
- CHEM225 Organic Chemistry 1 Module Week 1-4Dokument17 SeitenCHEM225 Organic Chemistry 1 Module Week 1-4Kezia CaratorNoch keine Bewertungen
- Chapter 2 Chemical Context of LifeDokument5 SeitenChapter 2 Chemical Context of Lifejohn doeNoch keine Bewertungen
- Intro To Organic ChemDokument91 SeitenIntro To Organic ChemMiguel Marquez GelacioNoch keine Bewertungen
- WaterDokument41 SeitenWaterWeihan ChiewNoch keine Bewertungen
- CHAPTER 4: Chemical BondingDokument25 SeitenCHAPTER 4: Chemical BondingHikmaNoch keine Bewertungen
- Carbon: The Backbone of Life: © 2011 Pearson Education, IncDokument28 SeitenCarbon: The Backbone of Life: © 2011 Pearson Education, IncMrLampNoch keine Bewertungen
- Test Bank For Microbiology Principles and Explorations 7th Edition by Black 2Dokument19 SeitenTest Bank For Microbiology Principles and Explorations 7th Edition by Black 2Ann OgoloNoch keine Bewertungen
- Physical Science Exam PDFDokument2 SeitenPhysical Science Exam PDFRemar Jhon PaineNoch keine Bewertungen
- Singapore-Cambridge GCE A-Level Chemistry Syllabus (2020Dokument58 SeitenSingapore-Cambridge GCE A-Level Chemistry Syllabus (2020KhoRogerNoch keine Bewertungen
- Kruss Tn306 enDokument8 SeitenKruss Tn306 enPlínio FurtatNoch keine Bewertungen
- Asphaltene Problems in ProductionDokument4 SeitenAsphaltene Problems in ProductionGanesh GanyNoch keine Bewertungen
- Unit 6 Test Study Guide: Molecular Polarity and Intermolecular ForcesDokument2 SeitenUnit 6 Test Study Guide: Molecular Polarity and Intermolecular ForcesDeepti SailappanNoch keine Bewertungen
- Inorganic Chemistry Notes on Chemical Bonding, Theories and Shapes of MoleculesDokument41 SeitenInorganic Chemistry Notes on Chemical Bonding, Theories and Shapes of MoleculesAshok MukhijaNoch keine Bewertungen
- Exam Short Answer and MatchingDokument17 SeitenExam Short Answer and MatchingElizabeth LeonNoch keine Bewertungen
- Solutions: Chemistry: A Molecular Approach, 2nd EdDokument145 SeitenSolutions: Chemistry: A Molecular Approach, 2nd EdFhadilla AmuNoch keine Bewertungen
- Integrated Pharmaceutics Lecture NotesDokument64 SeitenIntegrated Pharmaceutics Lecture NotesSolomonNoch keine Bewertungen
- Intermolecular Forces of AttractionDokument12 SeitenIntermolecular Forces of AttractionmochimochikoNoch keine Bewertungen
- Concise Inorganic Chemistry by J.D. LEEDokument13 SeitenConcise Inorganic Chemistry by J.D. LEEARNAV SINGHNoch keine Bewertungen
- 51a Chapter 1 2014 Copy 2Dokument37 Seiten51a Chapter 1 2014 Copy 2Efrain AnayaNoch keine Bewertungen
- C. Open-Mindedness: B. RationalityDokument26 SeitenC. Open-Mindedness: B. Rationalitycha yok100% (2)